AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/ 2690-8816/148
*Corresponding Author: H. Salar Amoli. Department of Chemistry, Amirkabir University of Technology, Tehran, IR: Iran.
Citation: F. Hadadzadeh1, S. A. Frozandeh1, H. Salar Amoli*1, (2024), Fabrication of a novel aptasensor based on paper carbon/gold nanoparticles/ reduced graphene oxide for early detection of hematuria, J Clinical Research Notes, 5(5); DOI:10.31579/ 2690-8816/148
Copyright: © 2024, Corresponding Author name. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 15 October 2024 | Accepted: 23 October 2024 | Published: 01 November 2024
Keywords: aprtasensor; hematuria; paper carbon; gold nanoparticles; reduced graphene oxide, urine.
Aptasensors are among the methods that can be used with high speed and accuracy for early and timely diagnosis of hematuria (early detection of blood in urine). The main objective of this research was a fast and accurate identification of red blood cells in urine. For these purposes, a new biosensor based on specific aptamer-hemoglobin, paper carbon (PC), gold nanoparticles (Au-NPs) and reduced grapheme oxide (rGO) have been designed and fabricated. UV-Vis spectroscopy, FT-IR, and scanning electron microscopy (SEM) have been used to investigate and characterize the synthesized materials. Modified electrodes have also been characterized using cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). Real urine samples containing hemoglobin and pure hemoglobin as reference, have been analyzed to evaluate the selectivity of the aptasensor and the fabricated sensor. The aptasensor is based on electrochemical methods, and the use of gold nanoparticles and reduced grapheme oxides in its design aims to enhance electron transfer, resulting in more accurate and reliable responses. The aptasensor exhibits the best performance at pH 7. The obtained response time was 25 min, and the detection limit of 0.05 µg/mL and a shelf life of 1 month has been achieved.
Hematuria is a common phenomenon, especially among children, and is often perceived as an incidental finding in urine tests. In such cases, initial symptoms go unnoticed, leading to acute complications. In these circumstances, children or adolescents with hematuria require radiographic imaging or initial urological interventions, such as cystoscopy [1].
Hematuria is a term derived from the Greek words "haima" meaning blood and "ouron" meaning urine. The presence of blood in urine cannot be visible to the naked eye and can only be observed under a microscope (microscopic hematuria). Hematuria is defined as the presence of five or more red blood cells (RBCs) after three consecutive freshly centrifuged samples in high-power fields. A positive urine test can be caused by myoglobinuria or hemoglobinuria, which can change the color of urine without any red blood cells being observed in microscopic evaluations [2-4]. Additionally, certain medications (sulfonamides, nitrofurantoin, salicylates, phenazopyridine, phenolphthalein), toxins (lead, benzene), and foods (food colorings, beets, rhubarb, berries, paprika) may falsely discolor urine. In infants, when urate crystals precipitate in the urine, a reddish or pinkish color may be seen in the diaper [5]. One of the most common causes of hematuria is lower urinary tract infection, particularly urinary tract and bladder infections (bladder stones). In older patients, the causes of this condition can be cancerous glands or prostatic hyperplasia. In younger patients, hematuria can be caused by kidney problems, and failure to timely diagnose it can lead to more significant issues [6].
Achieving success in treating diseases requires the development of methods for rapid and accurate detection. Accurate measurement and evaluation of diseases in clinical diagnosis are of utmost importance. Although there are multiple methods available and in use in this field, there is still a noticeable lack of suitable, rapid, selective, and cost-effective measurement and analysis methods. Electrochemical sensors, especially biosensors, offer attractive analytical characteristics and hold promising options for future clinical diagnostics. However, several issues that need to be addressed and resolved before these methods can be used for on-site diagnostics [7,8]. The design of biosensors is such that they can specifically react with a particular substance. The result of this reaction comes in the form of messages that microprocessor can analyze.
A biosensor can be defined as a quantitative or semi-quantitative analytical device that consists of a biological sensing element connected to or combined with a transducer, converting a biological signal into a measurable electrical signal. The construction of biosensors involves the expertise and experience of various scientific fields such as biochemistry, immunology, optical physics, electrochemistry, electronics, etc. [9].
The concept of biosensors was first introduced by Leland C. Clark in the early 1960s. He used an enzyme electrode to measure the concentration of glucose for diabetic patients, utilizing the enzyme glucose oxidase. The development of biosensors began in 1962 with the construction of an oxygen electrode by Clark at the Cincinnati Synthetics in the United States, for measuring the concentration of dissolved oxygen in blood. This sensor is also sometimes referred to as the Clark electrode. Later, by coating the electrode surface with an enzyme that aided in the glucose oxidation, this sensor was used for measuring blood glucose levels [10].
To comprehensively evaluate and assess the performance of biosensors, it's essential to consider multiple criteria. These include selectivity, limit of detection, precision, solution conditions, response time, pH sensitivity, operational lifetime, stability, and reproducibility [11].
Protein biomarkers are likely the most common type of biomarkers that are evaluated through molecular diagnostics. Various diseases can release specific proteins into the bloodstream or urine. These proteins can then be assessed by taking a blood or urine sample and subjecting it to a molecular diagnostic test. Some of the most important protein biomarkers for disease detection, especially cancer, include alpha-fetoprotein, cancer antigen 125, cancer antigen 153, cancer antigen 19, carcinoembryonic antigen, epidermal growth factor receptor, human epidermal growth factor receptor 2, interleukins, prostate-specific antigen, squamous cell carcinoma antigen, tumor necrosis factor-alpha, and vascular endothelial growth factor [ 12, 13]. However, in hematuria, blood is observed in the urine of the patient. The most common method for investigating hematuria is urine analysis. Urine analysis aims to detect red blood cells, protein, glucose, and any signs of inflammation or infection [ 14,15]. However, the common methods are time-consuming and prone to errors. In the field of biosensors, the interaction between biological receptors and analytes through thermal, optical, and electrochemical signals can be used for disease identification [16].
Aptamers, discovered in 1990, are single-stranded DNA or RNA sequences with high affinity for specific target molecules. They bind through a lock-and-key mechanism. Unlike antibodies, aptamers boast simplicity in production, reproducibility, and a diverse functional environment. Their numerous benefits, including cost-effectiveness and broad applications, make them valuable in biosensor design [17-20].
Recently, much attention has been focused on the development of nanomaterials in the production of biosensors. Nanomaterials are used as a platform for immobilizing biomolecules on surfaces, and on the other hand, nanoparticles can be attached to the sensing element and used for detecting or amplifying of various signals [21]. Nanoparticles have a very high surface-to-volume ratio, which increases their ability to interact with any surface. They exhibit unique physical and chemical properties at this scale that make them highly intriguing [22].
Also, in recent years, there has been a significant increase in the use of gold nanoparticles (AuNPs) for creating colorimetric, fluorescent, and electrochemical aptasensors. This is due to their unique characteristics, such as their large surface area, ease of synthesis and functionalization, good biocompatibility, and distinct electronic and optical properties. Various research groups have been focusing on combining the advantages of both AuNPs and aptamers to develop innovative aptasensors [23-25]. Scientists will utilize the stable immobilization and preservation of biological activity provided by gold nanoparticles. Furthermore, utilizing a nanostructured gold surface rather than a standard gold surface offers advantages such as enhanced electron transfer and a larger surface area for additional modifications. Previous investigations have successfully developed biosensors on screen-printed carbon electrodes that were modified with gold nanoparticles, demonstrating their effectiveness in detecting analytes at low concentrations [26, 27].
Recently various methods have been proposed for the measurement of hemoglobin using aptamers. These methods include sandwich assays [28] as well as label-free approaches [29]. However, the label-free assays developed thus far have not proven to be highly effective in detecting hemoglobin at low concentrations. In this study, we introduce a rapid method for measuring hemoglobin in urine, utilizing cyclic voltammetry (CV). Our biosensor incorporates gold nanoparticles (AuNPs) as an anchoring platform and a truncated aptamer with a high affinity for the target analyte [30]. In this research for the first time, a biosensor of aptamer-hemoglobin/ paper carbon/gold nanoparticle/ reduced graphene oxide has been fabricated for early detection of blood in urine.
2.1. Materials
Sodium citrate (Na3C6H5O7), chloroauric acid (HAuCl4), trimethylamine hydrochloride (NH2C(CH2OH)3. HCl), 2-amino, 2-hydroxy methyl, 1,3-propanediol (NH2C(CH2OH)3), disodium phosphate (Na2PO4), potassium dihydrogen phosphate (KH2PO4), potassium ferrocyanide (K4F e(CN)6), potassium ferricyanide (K3F e(CN)6), mercaptoethanol (C2H6OS), sulfuric acid (H2SO4, 98%), aniline (C6H5NH2), and hydrazine (N2H4) were procured from Sigma-Aldrich and utilized as received without any additional purification. Distilled water (DI water) obtained from Thermo Fisher, USA, was utilized in all conducted experiments. The Hb aptamer (Kd = 2.8 nM) was manufactured by Bio Basic Inc (Canada, https://www.biobasic.com). The aptamer with thiol modification is 5′HS− (CH2)6/ ACGCACACCAGAGACAAGTAGCCCCCCAAACGCGGCCACGGAACGCAGCACCTCCA TGGC -3′.
2.2. Apparatus
Fourier transform infrared (FTIR) spectrometry was conducted using a Nicolet Nexus 670 spectrometer from Thermo Fisher in the United States. A scanning electron microscope (SEM, MIRA3 TESCAN) was utilized to investigate the morphology of nanomaterials. Electrochemical studies, including cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and square wave voltammetry (SWV) were conducted using an Ivium Technologies potentiostat/galvanostat instrument from the Netherlands (https://www.ivium.com). The experiments were performed in a solution containing a 0.2 mM [Fe(CN)6]-3/-4 redox probe and 0.1 M PBS. All electrochemical tests were conducted using a three-electrode system. The working electrode consisted of a gold electrode with a radius of 1 mm. The counter electrode was made of platinum, and the reference electrode used was Ag/AgCl. Electrochemical impedance spectroscopy (EIS) tests were conducted over a frequency range of 10 kHz to 100 mHz. A perturbation amplitude of 0.22V was applied at the potential corresponding to the oxidation peak current observed in the cyclic voltammetry (CV) curves. All analyses were performed at room temperature.
2.3 Methods
2.3.1. Synthesis of rGO
The Hummers' method was used for the synthesis of graphene oxide. To begin the process, a glass container was placed inside an ice bath. The temperature was maintained 13°C throughout this stage, monitored using a thermometer. Then, 56 mL of 38% sulfuric acid was poured into the container, followed by the addition of 2 g of pure graphite. The mixture was stirred vigorously for 14 minutes. Subsequently, 6 g of potassium permanganate powder was slowly added to the mixture while stirring intensively for 1.5 hours. After completion, the stirring continued for an additional 30 minutes. The temperature of the bath was increased to 35°C, and after reaching thermal stability, stirring was continued for approximately one hour. To dilute the mixture, 100 mL of distilled water was cautiously added to the container, and the stirrer was turned off. Then, 300 mL of distilled water was poured into a larger container, and the contents of the initial container were slowly transferred to the larger one. Stirring was carried out for 30 minutes. The temperature of the bath should not exceed 40°C during this stage. After dilution with distilled water, 20 mL of 30% hydrogen peroxide was gently added to the container, and stirring continued for half an hour. At this stage, the color of the solution briefly changes to bright yellow. The mixture was then washed to remove ions and increase the pH by adding 10% HCl and distilled water, followed by stirring (centrifugation at 14,000 rpm for one hour). The container was then left undisturbed for approximately 4 hours to allow sedimentation of the materials. Subsequently, the mixture was placed in an oven at 50°C for 4 hours. The resulting powder is graphene oxide [31].
To prepare reduced graphene oxide, 3.0 mg/mL of graphene oxide was dissolved in 1 mL of distilled water. Then, 200 μL of aniline and 1 mL of hydrazine were added to the solution. The resulting solution was placed on a magnetic stirrer for 30 minutes and then heated at 100°C for one hour while stirring. Next, the desired reduced graphene oxide was synthesized, and to separate the remaining aniline and hydrazine, the solution was centrifuged, allowing the aniline and hydrazine to be separated from the reduced graphene oxide particles. It is worth mentioning that in this process, hydrazine acts as a reducing agent, while aniline serves as a catalyst.
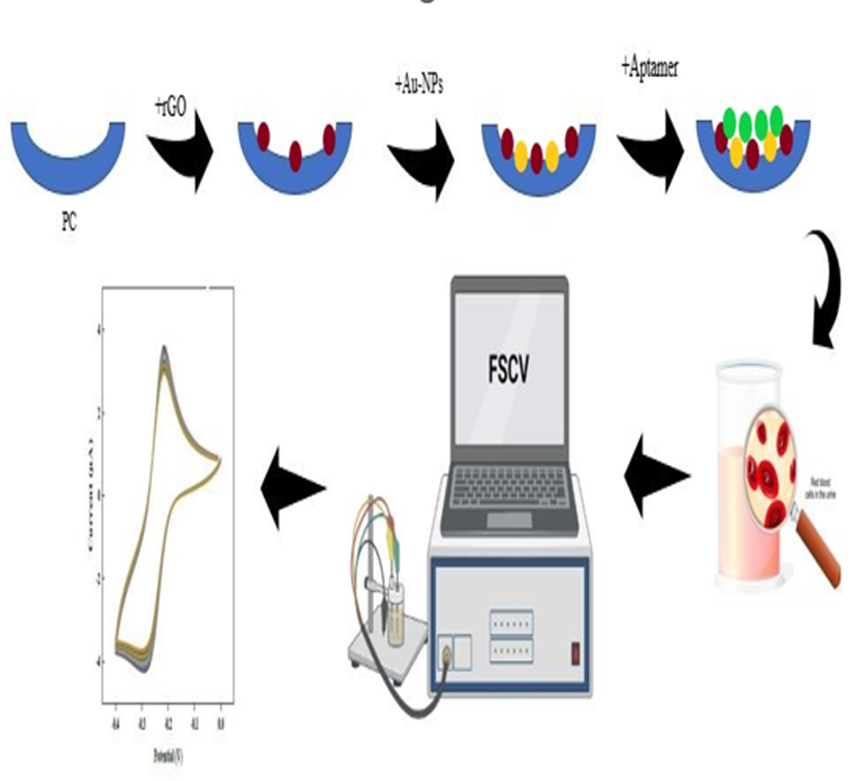
2.3.2. Synthesis of Au-NPs
Au-NPs are prepared by using a solution of Na3C6H5O7. The Na3C6H5O7 solution is prepared by dissolving 1 g of Na3C6H5O7 in 100 mL of distilled water. Then, 0.1 g of HAuCl4·3H2O is added to a round-bottom flask and the volume is brought to 100 mL with distilled water. The solution is then heated to 100 on a heater until it turns yellow. Once the HAuCl4·3H2O solution starts boiling, 2 mL of the prepared Na3C6H5O7 solution are added to it. At this stage, the gold particles are transformed into nanoparticles, and the solution turns purple within a few seconds. To further reduce the size of the gold colloid particles, the solution should be boiled for 17 minutes until the color changes from purple to red. After that, the gold solution is removed from the heater and allowed to cool to room temperature. To preserve the gold solution, it should be well covered to prevent any exposure to light.
2.3.3. Preparation of Aptasensor
To prepare the aptasensor buffer, 1.460 g of NaCl dissolved in 25 mL. distilled water. Then, 0.354 g of Na2HPO4 dissolved in 25 mL distilled water. These two solutions should be combined, and then the KH2PO4 solution should be added drop by drop. After preparing the mentioned solutions, the biosensor fabrication process is initiated. Drop casting method is used for the deposition of the prepared layers. After heating the carbon papers to purify them from any contamination, approximately 50 L of rGO solution is placed on it at room temperature and away from any light. Once the mentioned drop is dried, 50 L of Au-NPs solution is placed on it. This step is repeated once more, and then 10 L of aptamer buffer is also placed on it. After drying for 12 hours, 10 L of mercaptoethanol solution is placed on the deposited layer, the resulting aptasensor is washed with distilled water. To preserve the resulting aptasensor, it should be stored in aptasensor buffer solution at a temperature of 3. The resulting aptasensor has a shelf life of one month.
2.3.4. Real Sample Analysis
The analysis was conducted using human urine samples collected from Boghrat Laboratory (Tehran, Iran). The samples were subsequently stored in test tubes at a temperature of 3 °C until the time of testing. Before the analysis, all human blood samples were gently agitated at room temperature.
3.1. Characterization of Synthesized Materials
An FT-IR instrument was used to identify the functional groups of GO and rGO and confirm the synthesis of these materials. Fig. 1(a) shows the spectrum of GO. In this spectrum, we observe an absorption peak at 3400 cm-1. This absorption indicates the presence of the functional group O-H, which is characteristic of carboxylic acid [32,33]. The absorption peak at 2855 cm-1 and 2922 cm-1 respectively indicates the presence of symmetric and asymmetric CH2 groups. The absorption peak at 1627 cm-1 represents the C=C functional group of the unoxidized graphite groups [33,34]. The peak around 1721 cm-1 indicates the presence of the C=O functional group [35]. Fig. 1(b) represents the spectrum of rGO. According to this figure, the intensity of absorption peaks has decreased, but they have not completely disappeared. This means that the reduced graphene oxide has been synthesized correctly, but its reduction may not be 100% complete [36].
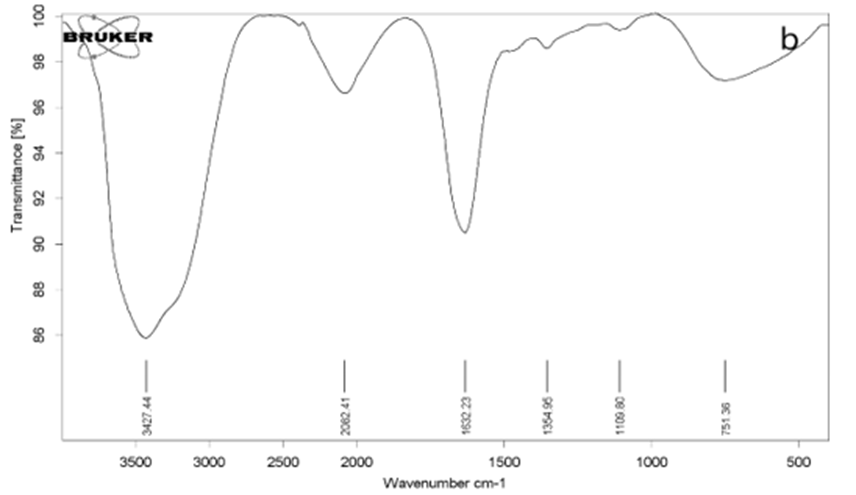
Fig. 1. FT-IR analysis of graphene oxide and reduced graphene oxide: a) GO, b) rGO, (b)
Also, UV-vis studies have been carried out. As Fig 2 illustrating, GO exhibits an absorption band at 230 nm, corresponding to the π - π * transition. However, for rGO, the peak shifts to 277 nm, indicating the removal of some functional groups on the surface of GO and the restoration of its structure [37,38]. The nanoparticles were prepared by reducing HAuCl4 with citrate as the reducing agent. As shown in Fig. 2(b), upon heating, the pale-yellow solution gradually changed to a dark purple color, confirming the production of gold nanoparticles. With increasing heating time, the solution gradually turned red. The color change is primarily due to light absorption and particle size. As the size of the nanoparticles decreases, they absorb shorter wavelengths of light, resulting in a reflected red color. The absorption peak also followed a similar trend. With increasing processing time, the absorption peak shifted towards shorter wavelengths, and we observe an absorption peak at 566 nm [39].
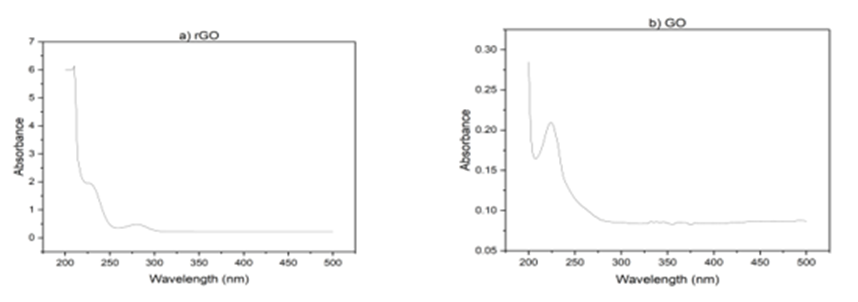
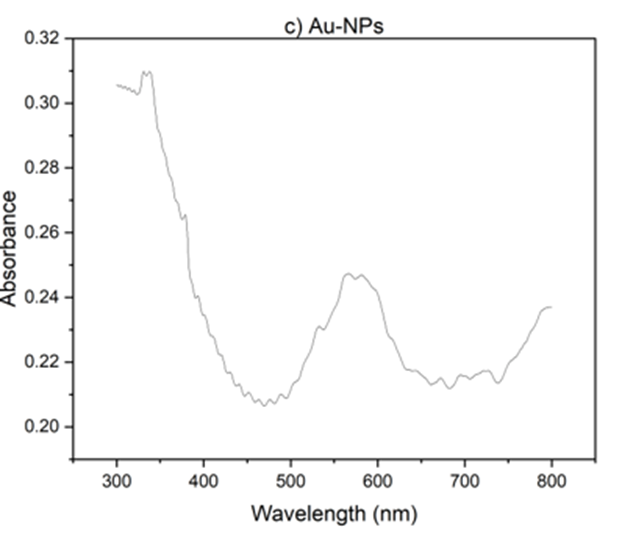
Fig 2. Uv- V is absorption spectra a) r GO, b) Go, C) Au-Nps
The morphological features of rGO, Au-NPs, and aptamers were meticulously examined using SEM, as presented in Fig. 3. In Fig. 3(a), discernible enhancement in the formation of rGO sheets is observed, highlighting improved porosity. Figs. 3(b) and 3(c) vividly illustrate the spherical configuration of gold nanoparticles, providing valuable insights into their dimensions. The successful synthesis of Au-NPs is evident based on the dimensions portrayed in the figure. Fig. 3(d) illustrates the surface morphology of the aptamer. In Fig. 2(e), although the aptamer is placed on the surface of gold nanoparticles, some areas with incomplete coverage allow the nanoparticles to be clearly seen. However, Fig. 2(f) shows complete coverage, where the larger size of the aptamer molecules compared to Au-NPs hinders the observation of the spherical particles The purpose of conducting the SEM test is to examine the uniform and distinct distribution of particles, which is essential for confirming the characteristics of the synthesized nanoparticles [40,41].

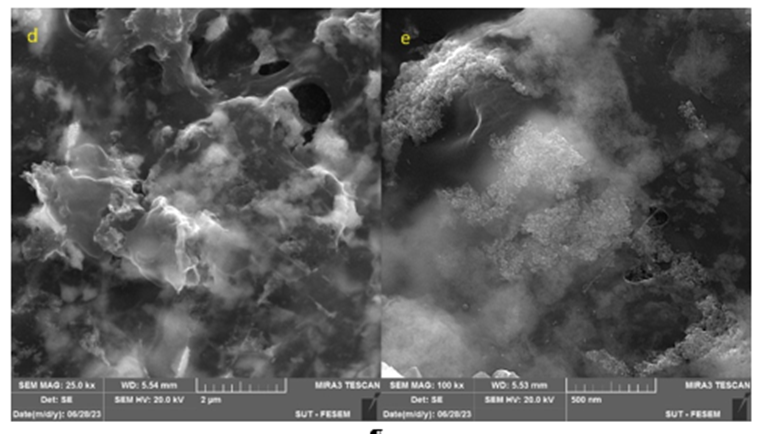
Fig. 3. SEM analysis: a) PC/rGO, b and c) PC/rGO/Au-NPs, d and e) PC/rGO/Au-NPs/Aptamer
3.2. Electrochemical Characterization
3.2.1. Characterization of sensing interface
Electrochemical impedance spectroscopy in the frequency range of 0.1 to 100,000 Hz was used to investigate the modified electrode. The studies involved rGO electrodes, rGO electrodes with Au-NPs, and aptamer-functionalized sensor in a 2.0 M ferrocyanide environment. The results of the electrochemical impedance spectroscopy test (Fig. 4) indicate that adding rGO increases the electrode surface resistance compared to the bare surface condition. Additionally, adding Au-NPs decreases the surface resistance, while adding aptamer and analyte increases the surface resistance again.
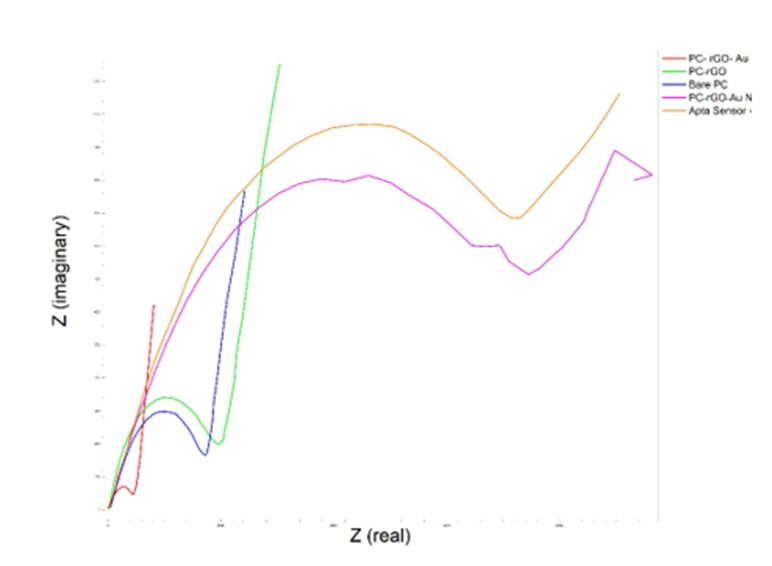
Fig. 4. Characterization of the synthesized materials using EIS
3.2.2. Investigation of Selectivity Level
To evaluate the performance and selectivity of the aptasensor towards hemoglobin, the experiment was conducted using three real urine samples containing hemoglobin, glucose, and fungus, respectively. SWV method was used for this purpose. According to the results in Fig. 5, the aptasensor's response to hemoglobin was faster, indicating the selectivity of the aptamer and, ultimately, the aptasensor. Given that real urine samples containing hemoglobin were used to determine the selectivity of the aptasensor, alternative interpretations of its results are possible. Urine samples containing hemoglobin were not interpretable to the naked eye, necessitating methods such as dipstick or microscopy to confirm the presence or absence of hemoglobin. Fortunately, all samples were obtained from pathology laboratories and were found to contain a trace amount of blood (1.0 mg/L).
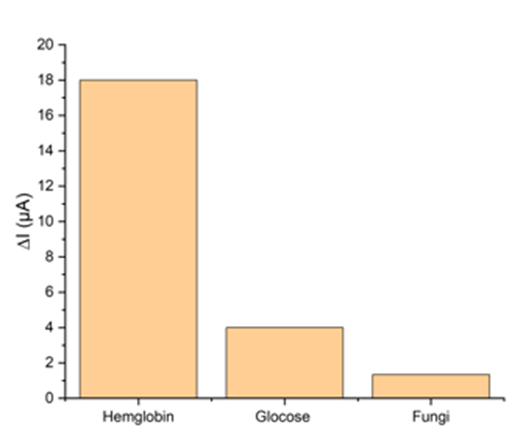
Fig. 5. Selectivity of the fabricated aptasensor (from left to right: hemoglobin, glucose, fungus)
3.2.3. Investigation of Concentration Effect
Cyclic voltammetry was used to determine the concentration effect on fabricated biosensor. As the analyte concentration increases, the electrochemical peak decreases because the resistance to electron transfer increases with increasing analyte concentration, and the maximum current decreases at each stage. Fig. 6 illustrates this phenomenon. Based on Fig. 6 data, it can be concluded that the detection limit of the aptasensor is 0.05 µg/mL.
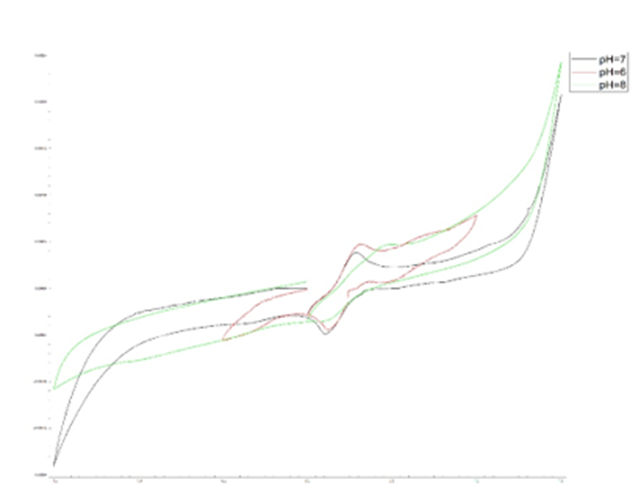
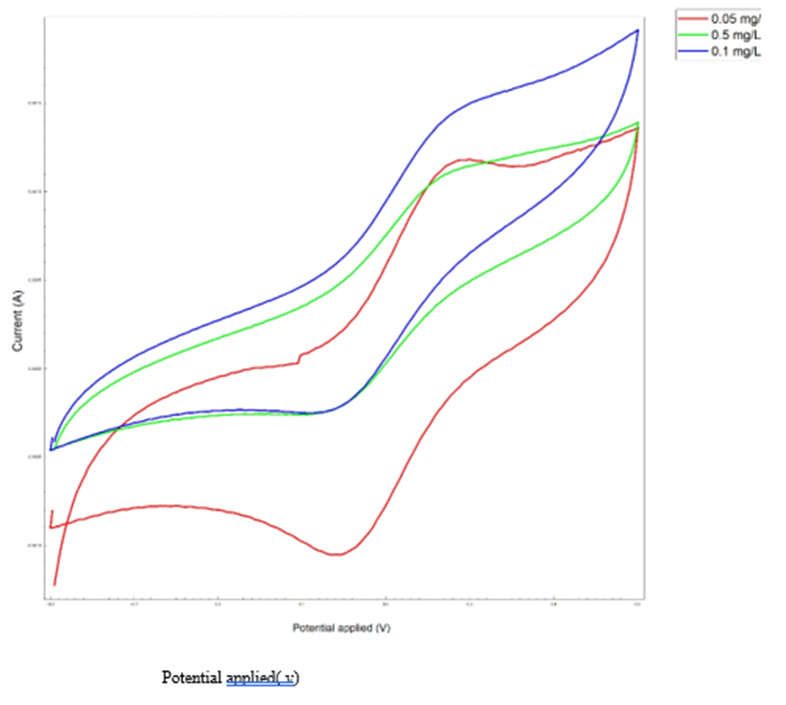
Fig. 6. Cyclic Voltametric Analysis. a) the effect of concentration (using real urine samples), b) the effect of pH
3.2.4. Aptasensor Response Time
To determine the optimal response time of the biosensor, a specific concentration of aptamer was used at different time intervals of 5, 15, 25, 35, and 45 minutes. Subsequently, CV was performed in the potential range of 0.2 to 0.5 V at a scan rate of 50 mV/s in a 2.0 mM ferrocyanide environment. According to the obtained results, at 5 minutes, the analyte's connection to the aptamer is negligible, indicating a very low absorption level. Additionally, the absorption level increased over time until 25 minutes, after which it remained relatively constant. These changes indicate that after 25 minutes, the analyte's absorption by the aptamer did not change significantly and is the optimal time. The fabricated aptasensor has a shelf life of one month and stable and reproducible until 6 successive uses without significant differences.
In the present research, a novel and advanced aptasensor was designed and fabricated for the detection of hematuria. Paper carbon, rGO, and AU-NPs were used to prepare this aptasensor. The developed aptasensor was also tested using actual urine samples, demonstrating its excellent selectivity and its ability to differentiate between normal and patient samples. Overall, this aptasensor was specifically designed to possess high sensitivity in medical facilities for the early detection of hematuria. The aptasensor exhibits the best performance at pH 7 and the detection limit of 0.05 µg/mL and a shelf life of 1 month has been achieved. The significant discoveries we made have the potential to drive progress in the critical area of hematuria detection.
Thanks to Amirkabir University of Technology for their support and encouragement to do this research work.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.
