AUCTORES
Globalize your Research
Review ariticle | DOI: https://doi.org/10.31579/2690-1897/254
Department of Pharmacology, School of pharmacy, RK University Rajkot, India.
*Corresponding Author: Kiran Dudhat, Department of Pharmacology, School of pharmacy, RK University Rajkot, India.
Citation: Chinmyee Saha, Ishita Zalavadiya, Kiran Dudhat, (2025), Emphasis on the In-Vivo & In-vitro Screening Models for the Evaluation of Disease Acting on Alzheimer’s Disease, J, Surgical Case Reports and Images, 8(5); DOI:10.31579/2690-1897/254
Copyright: © 2025, Kiran Dudhat. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 08 May 2025 | Accepted: 19 May 2025 | Published: 27 May 2025
Keywords: neurodegenerative disorder; in-vitro model; in-vivo model; evaluation; screening
Alzheimer's disease is a neurodegenerative disorder with cognitive impairment and pathological hallmarks. Alzheimer’s disease causes the brain to shrink and brain cells to eventually die. Alzheimer’s disease is the most common cause of dementia. Therapeutic interventions are challenging, and in vivo and in vitro screening models are valuable tools for evaluating drug candidates. These models evaluate cognitive performance, neuropathological changes, and molecular biomarkers, providing a comprehensive understanding of drug efficacy. In vivo models, like transgenic mouse models with Alzheimer's disease genetic mutations, allow researchers to study drug effects within a complex biological system, evaluating cognitive performance, neuropathological changes, and molecular biomarkers. In vitro models, like cell-based assays, neuronal cultures, and organoids, provide insights into drug mechanisms, toxicity profiles, and selectivity, and facilitate high-throughput screening for large compound libraries and potential lead molecules. The review article highlights the significance of in vivo and in vitro screening models in drug discovery and development, particularly in preclinical research for evaluating Alzheimer's disease drugs. In vivo and in vitro screening models for Alzheimer's disease drugs have significantly enhanced understanding and therapeutic interventions, aiding in screening compounds, assessing efficacy, and optimizing lead candidates for further research.
Alzheimer's disease is a progressive neurodegenerative disorder affecting around 50 million people worldwide, causing a decline in memory, executive function, and personality change. It is named after Alois Alzheimer and results in synapse loss and neuronal atrophy, characterized by amyloid plaques and neurofibrillary tau tangles. Both genetics and environmental factors are believed to play a role in AD, with most cases being sporadic and having no single genetic cause. Environmental and metabolic risk factors include diabetes, cerebrovascular disease, poor diet, head injury, and stress. The amyloid hypothesis, which suggests AD begins and progresses, leaves many questions, including the best drug target and the upstream cause of the rise in amyloid-β in sporadic cases. There is still a lack of understanding of how AD develops and therapies to help individuals combat the disease [1-7]
1.2. Types of Alzheimer's Disease
Early-onset Alzheimer's (EOA)
Those under65, frequently in their 40s or 50s, are susceptible to early-onset Alzheimer's. As little as 5% of all cases of Alzheimer's disease demonstrate this rarity. It is more prevalent in those who have Down syndrome and is connected to changes in the brain like plaques, tangles, and loss of volume. [8]
Late-onset Alzheimer's (LOA)
The most prevalent type of Alzheimer's disease, known as late-onset Alzheimer's (LOA), affects individuals 65 years of age and older and has no known genetic cause or family history [8]
Familial Alzheimer's disease (FAD)
A genetically linked form of Alzheimer's disease, familial Alzheimer's disease (FAD) affects at least two generations in affected families and accounts for less than 1% of cases overall [8]
1.3. Etiology
Aβ precursor protein
Genes exhibiting autosomal dominant mutations that cause Alzheimer's disease first appear in the Aβ precursor protein (APP). Ten percent of cases that are familial are caused by more than fifty known APP mutations, most of which are centered around β and γ-secretase cleavage sites. Numerous mutations, according to research, raise Aβ synthesis, which promotes amyloid buildup. Promoter mutations or APP duplications may in rare instances result in AD [9,10] .
Presenilin
Autosomal dominant AD is caused by the γ-secretase catalytic components encoded by presenilin 1 and 2. Thirty to fifty percent of cases of familial EOAD are due to variants in PSEN1. Based on research, mutations in PSEN1 and PSEN2 change the production of Aβ but result in a loss of function.
Other genetic risk factors
A gene's susceptibility to Alzheimer's disease is associated with variations in TREM2, APOE, CLU, BIN1, SORL1, and PICALM. The most frequent risk factor, the E4 allele of APOE, triples risk. The uncommon variation TREM2R47H contributes to inflammation in the pathophysiology of AD. [11-13]
Down syndrome
By the age of 65, dementia affects up to 80% of people with Down syndrome (DS) [14]. Like other cases of EOAD, even at a young age of less than 40, amyloid and tau pathology start considerably earlier than in LOAD [15–16]. when compared to LOAD, amyloid and tau pathology start sooner. Gene triplication may also contribute to DS, which is caused by chromosome 21 trisomy and elevated Aβ levels. [14, 18-19]
Inflammation
Brain hypoperfusion and inflammation are common in sporadic Alzheimer's disease (AD), which is a result of a combination of genetic and environmental factors [20, 21]. Traumatic brain injury and bone fractures increase the risk of dementia. A faster rate of cognitive decline is linked to higher levels of inflammatory markers as well as activated microglia and astrocytes in patients with AD. [23]
Cerebral, cardiovascular disease and diabetes
An elevated risk of Alzheimer's disease (AD) has been associated with vascular disease, including cardiovascular and cerebrovascular diseases [24]. Diabetes doubles the risk of dementia, and other lifestyle factors like obesity, high cholesterol, poor diet, and sedentary behavior also increase the risk. [25,27]
1.4 neuropathology
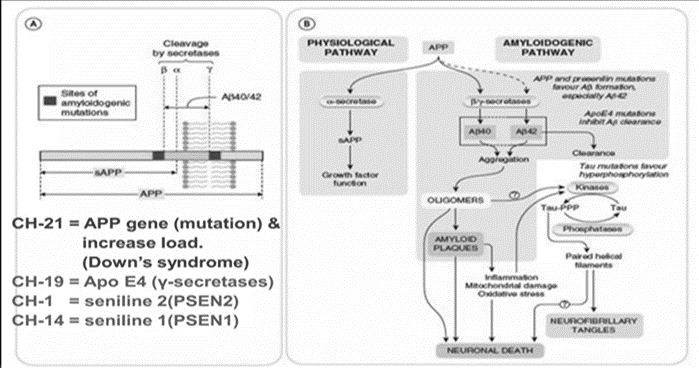
Figure 1: Alzheimer's Neropathology [28]
Aβ plaques
The brain is affected by senile plaques, which are composed of resistant Aβ sheets formed by amyloid peptides that co-localize with microglia and neuronal debris. In older people with normal cognitive function, they can be significant and come before tau. (29–31), but other research indicates
a link between dementia severity and cognitive decline. Perhaps a more accurate indicator of disease would be variations in the concentration of Aβ oligomers. (32-23). When Aβ pathology precedes cognitive changes, individuals with high plaque levels who are cognitively normal may have "prodromal" disease. (34, 35).

Figure 2: (A) Thioflavin S staining of Aβ plaques in the cortex of CRND8 APP transgenic mouse. (B) AT8 staining of NFTs within an aged human CA1 region- hippocampus. (C) Spread of amyloid and tau throughout the brain[36]
Neuronal fibrillary tau tangles:
Alzheimer's disease and other neurodegenerative diseases are associated with hyperphosphorylated tau protein aggregates, or NFTs. They diffused across the brain, producing toxicity and loss of neurons, but they had little association with deteriorating cognitive function. Confirmation and testing are necessary for diagnosis. [37,38]
1.4 Pathogenesis
Aβ and APP
Alzheimer's disease (AD) may result from mutations in the APP and presenilin genes, according to the amyloid hypothesis. The development, growth, and survival of neurons are influenced by both APP and Aβ, with the accumulation of Aβ possibly playing a role in AD. [39,40]
NFTs and Tau
A key player in neurodegenerative diseases such as Alzheimer's disease is the cytoskeletal protein tau. Neurotoxic effects from mutations can include altered axonal transport, transcriptional changes, and increased degeneration. Together, tau and Aβ result in transcriptional deficiencies and alterations to the synapses in AD. [41]
Mitochondrial dysfunction and oxidative stress
When mitochondrial function is disrupted by Alzheimer's disease, cytochrome oxidase activity is lowered, oxidative stress increases, and changes in mitochondrial morphology, number, and transport occur. These effects are both hereditary and environmental. While transgenic APP mice show increased Aβ within synaptic mitochondria, causing dysfunction and oxidative stress, Thy-1-APP mice show reduced membrane potential and increased ROS production [44]
Insulin
Oxidative stress and energy deficiencies can result from insulin resistance, reduced receptors in the Alzheimer's disease (AD) brain, and late-stage diabetes. Insulin is involved in neurotransmission and has the ability to shield the nervous system from damage [46]. In mice, levels of Aβ and β-secretase are elevated by insulin and metabolic inhibitors. [47]
Hypoglycaemia and vascular dysfunction
Alterations in metabolic proteins, glucose receptors/transporters, or hypoglycemia brought on by excessive medication may be the cause of the connection between diabetes and Alzheimer's disease. Tachycardia, increased oxidative stress, and neuronal death may result from this. [48]
Inflammation
Alzheimer's patients rapidly deteriorate as a result of inflammation, which has a significant impact on the disease. While it first shields neurons, it can also lead to tau pathology, increased Aβ load, neurotoxicity, and ROS levels. [49-52]
Ubiquitin-proteasome system
Through an enzymatic process, the ubiquitin-proteasome system (UPS) degrades excess and misfolded proteins, which is essential for synapse function. Targeting monomeric proteins may prevent proteasome activity[53], which could result in a hazardous accumulation of extra proteins in the brain.
Autophagy lysosome pathway
An involvement of autophagy in tau clearance and Aβ generation is suggested for AD pathogenesis, along with lysosomal dysfunction. BIN1, SORL1, and PICALM are among the genes linked to AD that may have a direct impact on APP endosomal processing. [55,56]
Cholinergic hypothesis
Linking acetylcholine with other pathologies is still difficult, but the cholinergic hypothesis—which was first proposed in AD—suggests aberrant levels of acetylcholine in the brain, affecting cholinergic neurons and affecting Aβ accumulation [57]. In areas where plaques and tangles are present, pyramidal neurons—which are mainly glutamatergic—are lost. [58].
1.5. Mechanism of Alzheimer Disease

Figure 3: Enzyme acts on APP (Amyloid Precursor Protein) 7 cut into in fragments. The beta amyloid fragments is crucial in the formation of sanile plaques in AD disease [59]
Aβ hypothesis:-
Neurotoxicity, senile plaques, and the clinical manifestations of AD are caused by increased production of Aβ fiber. Plaques are caused by the breakdown of APP into β-amyloid fragments by enzymes after it penetrates the neuronal membrane.
Tau hypothesis:-
Healthy neurons have microtubules, which act as tracks for nutrients and molecules. In Alzheimer's Disease (AD), tau, a protein, becomes tangled, leading to microtubule disintegration and collapsing the neuron's transport system, potentially causing communication malfunction and cell death.

Figure 4: Dendrite disinteration in Las protein tau [59]
1.6 Current treatment for Alzheimer disease
• Acetylcholine esterase inhibitors (AChEIs) [60]
Tacrine, Donepezil, Galantamine, Rivastigmine & Huperzine A
• NMDA receptor Antagonist [60]:
Memantine

Table 01: Details about current using drugs for AD [(60]
1.7 Limitation of conventional drugs available for Alzheimer Drug:
Alzheimer's medications, like cholinesterase inhibitors and memantine, provide temporary relief but do not address the underlying cause or slow its progression. Their effectiveness varies among individuals, with modest long-term effects. Current medications do not prevent or reverse Alzheimer's disease progression, and their use is limited to mild to moderate stages, with no FDA-approved medication for severe Alzheimer's.
1.8 Importance of drug development in Alzheimer disease:
Alzheimer's disease has no cure and current treatments provide temporary relief. Drug development is crucial due to the increasing number of affected individuals and lack of curative treatments. A curative treatment could improve the quality of life for millions by eliminating physical, emotional, and financial care. Drug development can also enhance our understanding of the disease, revealing its underlying causes and improving diagnosis and treatment precision.
1.9 Alzheimer’s drugs in development
Aducanumab:
Aducanumab targets beta-amyloid protein in the brain, causing AD symptoms by preventing messages from being sent between cells. [61]
Solanezumab:
Solanezumab, an anti-amyloid drug, is being studied for its potential to slow cognitive decline in individuals with Alzheimer's disease who do not yet show memory loss or thinking difficulties. [61]
Insulin:
The Study of Nasal Insulin in the Fight against Forgetfulness (SNIFF) is investigating if a nasal spray type of insulin can enhance memory function in individuals with mild memory issues or Alzheimer's disease. [61]
Others:
Current drugs include verubecestat, AADvac1, CSP-1103, and intepirdine, but AD is unlikely to be cured by a single medication, focusing on prevention and treatment. [61]
1.10Alternative treatments for Alzheimer’s disease
Coconut oil
Caprylic acid, found in processed coconut oil, is used in Ketasyn, a drug that improves memory performance and reduces cognitive decline [61]
Omega-3 fatty acids
Omega-3 fatty acids, found in fish, nuts, and oils, may reduce cognitive impairment. [61]
Coral calcium
Coral calcium, derived from seashells and sea life, is a popular calcium supplement for treating AD. [61]
Herbal medicine
Ginkgo biloba, an herb with anti-inflammatory and antioxidant properties, may benefit people with cognitive impairment caused by AD. Choto-san, an herbal mixture with 11 medicinal plants, has been used to treat dementia, improving memory and learning. Kami-untan-to, a Japanese herb, has been found to improve nerve growth in rat brain cells, potentially slowing AD [61]
1.11 Some Novel Targets for Alzheimer’s disease treatment:
Tau aggregation inhibitors:
Antibodies prevent abnormal tau protein aggregation into tangles, aiming to stabilize its normal functional form.
Kinase inhibitors:
Tau phosphorylation is involved in its abnormal aggregation, and inhibiting specific kinases involved in tau phosphorylation can prevent tau pathology and cognitive decline.
Anti-inflammatory approaches:
Inflammation is believed to contribute to AD progression, and targeting neuroinflammation with anti-inflammatory drugs or immune-modulating therapies may help reduce tau pathology.
Apolipoprotein E (APOE):
Certain variants of the APOE gene are linked to an increased risk of Alzheimer's.
Tau degradation enhancers:
The brain's ability to clear abnormal tau is enhanced by boosting the activity of cellular degradation pathways like autophagy or the ubiquitin-proteasome system.
Epigenetic modifications:
Modulating epigenetic processes to regulate gene expression and potentially reverse or slow down Alzheimer's progression.
Microglia activation:
Modulating the activation and function of microglia, the brain's immune cells, to maintain a healthy brain environment.
1.12. How screening methods are useful to screen test drugs
Screening methods help researchers efficiently test numerous compounds for desired pharmacological activity, identifying active compounds, assessing potency and selectivity, evaluating drug-drug interactions, assessing safety and toxicity profiles, and optimizing lead compounds. They measure drug potency by comparing it to the target or disease model, ensuring the drug interacts specifically with the intended target. High-throughput screening (HTS) methods expedite drug discovery by screening thousands to millions of compounds, often using robotic systems and automation to enhance efficiency and reduce human error.
1.13 Brief Information on the overall Principle behind the screening models:
Drug screening models are used by researchers to replicate Alzheimer's disease pathology using genetically modified animal models or cultured cells. They identify molecular targets associated with Alzheimer's disease and test their ability to modulate these targets using existing drugs, natural products, or synthesized molecules. These models evaluate the efficacy of potential Alzheimer's drugs by measuring their impact on disease-related markers and assessing safety and pharmacokinetic properties. Promising compounds identified through screening models can progress to preclinical studies and clinical trials, providing valuable insights into therapeutic effects and safety considerations.
2.1. Inhibition of Acetylcholine-esterase activity in rat striatum
2.1.1.PURPOSE AND RATIONALE:-
This assay screens drugs for acetylcholine-esterase inhibition, potentially useful for Alzheimer's disease treatment, as AChE is responsible for rapid acetylcholine hydrolysis and inactivation. [62]
2.1.2.PROCEDURE:-
Reagents:-
The process involves preparing a phosphate buffer, a substrate, a DTNB, and a stock solution of the test drug, which are serially diluted to a final concentration of 10-4 M. [62]
Tissue Preparation:-
Male Wistar rats are decapitated, brains removed, dissected, and homogenized. A suspension is added to vehicle or test drug, and re-incubated at 37°C. [62]
Assay:-
The Beckman DU-50 spectrophotometer measures enzyme activity, and reagents are added to blank and sample cuvettes & also ,Blank: 0.8ml PO4 buffer/DTNB 0.8ml buffer/Substrate ,Control: 0.8ml PO4 buffer/DTNB/Enzyme 0.8ml PO4 buffer/Substrate ,Drug: 0.8ml PO4 buffer/DTNB/Drug/Enzyme 0.8ml PO4 buffer/Substrate [62]
2.1.3. EVALUATION:-
The substrate concentration is 10 mM diluted 1:2 in an assay to produce a final concentration of 5 mM, which is used for IC50 calculations. The concentration of DTNB is 0.05 mM, resulting in a final concentration of 0.05 mM.
• % Inhibition=slope control−slope drug ×100 slope control,• IC50 values are calculated from log-probit analysis. [62]
Human Serum
2.2.1. PURPOSE AND RATIONALE:-
The substrate concentration is 10 mM diluted 1:2 in an assay to produce a final concentration of 5 mM, which is used for IC50 calculations. The concentration of DTNB is 0.05 mM, resulting in a final concentration of 0.05 mM.. [62]
2.2.2.PROCEDURE:-
Reagents
Add 0.05M phosphate buffer, pH7.2, 6.85g NaH4PO4·H2O/100ml distilled H2O, and 13.40g NaH4PO4·H2O/100ml distilled H2O until pH reaches 7.2. Substrate in buffer (225.8mg s-butyrylthiocholine chloride) q.s. to 100ml with 0.05M phosphate buffer, pH7.2.The drug is serially diluted with 0.5mM DTNB in a buffer, followed by a 2mM stock solution of the test drug in a solvent. [62]
Enzyme Preparation:-
Lyophilized human serum is reconstituted in distilled water, added to test drug, and pre-incubated at 37°C for 10 minutes.
Assay:-
Enzyme activity is measured with the Beckman DU50 spectrophotometer.Reagents are added to the blank and sample cuvettes: Blank: 0.8ml PO4 buffer/DTNB 0.8ml buffer/Substrate.Control: 0.8ml PO4 buffer/DTNB/Enzyme 0.8ml PO4 buffer/Substrate.Drug: 0.8ml PO4 buffer/DTNB/Drug/Enzyme 0.8ml PO4 buffer/Substrate 26 [62]
2.2.3 EVALUATION:-
For IC50 determinations:- Substrate concentration is 10mM diluted 1:2 in assay yielding final concentration of 5mM. DTNB concentration is 0.5mM yielding 0.25mM final concentration[62]
3.% Inhibition= slope control−slope drug × 100 slope control,,IC50 values are calculated from log-probit analysis
2.3. Stimulation of Phosphatidylinositol Turnover in Rat Brain Slices
2.3.1.Principle:
Phosphatidylinositol turnover in rat brain slices is a biochemical assay assessing signaling pathways, measuring inositol phosphate production as a reflection of PI turnover. [62]
2.3.2. Procedure:
Rat brain slices are prepared by dissecting and slicing it into thin sections using a specialized tissue slicer or microtome, then incubated in a suitable buffer to maintain viability. Brain slices are stimulated with neurotransmitters, hormones, or pharmacological agents to activate the phosphoinositide signaling pathway, thereby affecting the turnover of PI enzymes.Brain slices are stimulated, incubated for PI turnover, and harvested for lipid extraction, including PI and its metabolites.The extracted lipids are analyzed using HPLC or radioisotope-based assays to measure IP levels, which indicate PI turnover and the activity of the phosphoinositide signalling pathway. [62]
2.3.3. Evaluation:
The study uses inositol phosphates (IP) levels in brain slices to understand phosphoinositide turnover and signaling pathway activation. It uses dose-response analysis to determine sensitivity and potency, and time-course analysis to identify peak activity or sustained responses. The study suggests that adding specific inhibitors or activators of key enzymes or receptors can help investigate the signaling pathway's mechanisms. [62]
Reagents
Add 0.05M phosphate buffer, pH7.2, 6.85g NaH4PO4·H2O/100ml distilled H2O, and 13.40g NaH4PO4·H2O/100ml distilled H2O until pH reaches 7.2. Substrate in buffer (225.8mg s-butyrylthiocholine chloride) q.s. to 100ml with 0.05M phosphate buffer, pH7.2.The drug is serially diluted with 0.5mM DTNB in a buffer, followed by a 2mM stock solution of the test drug in a solvent. [62]
Enzyme Preparation:-
Lyophilized human serum is reconstituted in distilled water, added to test drug, and pre-incubated at 37°C for 10 minutes.
Assay:-
Enzyme activity is measured with the Beckman DU50 spectrophotometer.Reagents are added to the blank and sample cuvettes: Blank: 0.8ml PO4 buffer/DTNB 0.8ml buffer/Substrate.Control: 0.8ml PO4 buffer/DTNB/Enzyme 0.8ml PO4 buffer/Substrate.Drug: 0.8ml PO4 buffer/DTNB/Drug/Enzyme 0.8ml PO4 buffer/Substrate 26 [62]
2.2.3 EVALUATION:-
For IC50 determinations:- Substrate concentration is 10mM diluted 1:2 in assay yielding final concentration of 5mM. DTNB concentration is 0.5mM yielding 0.25mM final concentration[62]
3.% Inhibition= slope control−slope drug × 100 slope control,,IC50 values are calculated from log-probit analysis
2.3. Stimulation of Phosphatidylinositol Turnover in Rat Brain Slices
2.3.1.Principle:
Phosphatidylinositol turnover in rat brain slices is a biochemical assay assessing signaling pathways, measuring inositol phosphate production as a reflection of PI turnover. [62]
2.3.2. Procedure:
Rat brain slices are prepared by dissecting and slicing it into thin sections using a specialized tissue slicer or microtome, then incubated in a suitable buffer to maintain viability. Brain slices are stimulated with neurotransmitters, hormones, or pharmacological agents to activate the phosphoinositide signaling pathway, thereby affecting the turnover of PI enzymes.Brain slices are stimulated, incubated for PI turnover, and harvested for lipid extraction, including PI and its metabolites.The extracted lipids are analyzed using HPLC or radioisotope-based assays to measure IP levels, which indicate PI turnover and the activity of the phosphoinositide signalling pathway. [62]
2.3.3. Evaluation:
The study uses inositol phosphates (IP) levels in brain slices to understand phosphoinositide turnover and signaling pathway activation. It uses dose-response analysis to determine sensitivity and potency, and time-course analysis to identify peak activity or sustained responses. The study suggests that adding specific inhibitors or activators of key enzymes or receptors can help investigate the signaling pathway's mechanisms. [62]
3.IN-Vivo Screening Models:
3.1. Step-down method:
3.1.1. Purpose and Rationale:
An animal, such as a mouse or rat, often explores a rectangular compartment by stepping down to the floor when placed on an elevated platform.[62]
3.1.2. Procedure:
Requirements: A) Either sex of mice or rats are employed. B) Over the block, a rectangular box (50 by 50) with an electrifiable grid floor 35 fits. C) The shock device and grid floor are linked. [62]
A typical paradigm consists of : a) Familiarization b) Learning c) Retension test
A)Familiarization:
Animal is positioned on the platform. after the cylinder is raised, released Measured is the latency to descend It is brought back to the home cage after 10 seconds of expolaration. [62]
B) Learning:
The animal has immediately lowered itself from the platform. An unnecessary shock is given (foot shock: 50Hz: 1.5 mA; 1 sec). The creature goes back to its own prison. [62]
C) Retension test:
One day following the educational journey Step-down delay is measured once the animal is positioned back on the platform. Once the animal descends or stays on the platform (60 seconds is the cut-off time), the test is considered completed. [62]
3.1.3. Evaluation:
Measured are the descent times during the learning phase and the retention test. Learning is characterised as an extension of the step-down latency.. [62]
3.2. Step-through Method
3.2.1. Purpose and Rationale:-
This test uses the normal behavior of mice and rats. These animals avoid bright light and prefer dim lighting. When placed in a brightly lit space connected to a dark enclosure, they rapidly enter the dark compartment and remain there.[62]
3.2.2. Procedure:-
The test apparatus consists of a small room connected to a large dark room through a guillotine door. The small compartment is illuminated by a 7 W/12 V bulb. In the acquisition test, animals are placed in the illuminated compartment at the maximum distance from the guillotine door and the latency to enter the dark compartment is measured. Animals that do not pass through the door within the cutoff time of 90 seconds (mice) or 180 seconds (rats) are not used. Immediately after the animal enters the darkroom, the door is automatically closed and the inevitable foot shock is administered. The animals are then promptly removed from the apparatus (within 10 seconds) and returned to their home cages. The test procedure is repeated with or without drug; the cutoff time on day 2 is 300 seconds (mice) or 600 seconds (rats), respectively. [62]
3.2.3. Evaluation:-
The study measures step-through time during the learning phase and retention test, focusing on prolonging step-through latencies specific to the experimental situation, and defining learning as an increase in step-through latency. [62]
3.3. Scopolamine –Induced Amnesia In Mice
3.3.1. Purpose and Rationale:
The muscarinic inhibitor scopolamine causes transient amnesia in young volunteers and mice, and several cholinergic drug agonists have been found to reverse this amnesia.[63]
3.3.2. PROCEDURE:
The scopolamine test is conducted on bunches of 10 male NMRI mice weighing between 26-32 g in a single trial. After regulating 3mg/kg of scopolamine hydrobromide through intraperitoneal infusion, each mouse is separately set within the brightly lit segment of a two-chambered device. Taking after a brief introduction period, the mouse enters the moment, darker chamber. Once interior the moment chamber, the entryways are closed to avoid elude. A 1 mA, 1-second foot stun is at that point connected through the network floor. The mouse is at that point returned to its domestic cage. After 24 hours, the testing is rehashed by placing the creature back within the brightly lit chamber. The idleness, or time taken, for the mouse to enter the dim chamber inside a 5-minute test session is measured electronically. In contrast, untreated control creatures enter the darker chamber within the moment trial with a idleness of around 250 seconds. Treatment with scopolamine diminishes the idleness to 50 seconds. Test compounds are managed 90 minutes some time recently the preparing session. The drawn out inactivity shows that the creature recalls being rebuffed and, as a result, maintains a strategic distance from the darker chamber...[63]
3.3.3. Evaluation:
The areas of the test drugs were measured after administration of various doses, with some drugs showing linear dose-response curves and others showing a U-shaped response.[63]
3.4. Ischemia Induced Alzheimer Disease Screening Model
3.4.1 Principle:
Research models of ischemia-induced Alzheimer's disease study brain ischemia and its impact on development and progression by modeling ischemic conditions and assessing cognitive impairment and pathological changes. [63]
3.4.2. Procedure:
Cerebral ischemia in rats using methods such as bilateral carotid artery occlusion, middle cerebral artery occlusion, or global cerebral ischemia reduces blood flow and releases oxygen. The duration and severity of ischemic stroke are determined by careful management and monitoring. Methods such as cerebral blood flow measurements, EEG monitoring or histological analysis are used to ensure homogeneity between the experimental groups. Cognitive function was evaluated by postmortem behavioral tests, including memory, learning, attention, and object recognition, comparing animals with the number 039. Pathological diagnosis uses methods such as immunohistochemistry, ELISA analysis, and Western blotting to evaluate the pathological changes of Alzheimer's disease, including amyloid beta plaques, tau protein chains, neuroinflammation, synapse loss, and neuronal damage. Ischemia-induced Alzheimer's disease models can assess the efficacy of therapeutic interventions targeting specific aspects of the disease, such as beta-amyloid aggregation, tau phosphorylation, or neuroinflammation... [63]
3.4.3 Evaluation:
In this study, we evaluate a diagnostic model of ischemia-induced Alzheimer's disease using animal behavioral tests, pathological changes, neurochemical and molecular analyses, and evaluate interventions in medicine. By comparing cognitive impairment and pathological changes in ischemic and control groups, we will assess the consistency and effectiveness of medical interventions and determine their therapeutic value [63]
The review article emphasizes the types, etiology , neuropathology, mechanism of Alzheimer’s disease, importance of screening of new therapeutic component for this disease, details procedures & significances of in-vivo and in-vitro screening models in evaluating Alzheimer's disease drugs .Also neuropthology,, mechanism of this disease are described here with proper figures. Besides these the In-vivo models, provide a comprehensive approach to studying drug effects in living organisms, while in-vitro models, like cellular and biochemical assays, offer insights into molecular and cellular mechanisms. However, these models may not fully represent the complexity of Alzheimer's disease or the in vivo environment. Combining these models is crucial for drug development.