AUCTORES
Globalize your Research
Case Report | DOI: https://doi.org/10.31579/2641-0419/498
Director Advanced Cardiac Imaging and Echocardiography Laboratory Cardiology Section, Specialty Department Miami VA Healthcare System.
*Corresponding Author: Alex P. Rodriguez, Director Advanced Cardiac Imaging and Echocardiography Laboratory Cardiology Section, Specialty Department Miami VA Healthcare System.
Citation: Alex P. Rodriguez, Abigail Qin-Nelson, Muzammil Musani, Alan Moorman, Terry Bauch, (2025), Echoes, Shadows, and Scans:A Multimodality Pursuit Unmasking a Peripartum Sinus of Valsalva Rupture, J Clinical Cardiology and Cardiovascular Interventions, 8(11); DOI:10.31579/2641-0419/498
Copyright: © 2025, Alex P. Rodriguez. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 08 July 2025 | Accepted: 22 July 2025 | Published: 29 July 2025
Keywords: sinus of valsalva aneurysm rupture; peripartum heart failure; multimodality imaging; cardiac mr; shunt; qp:qs
Sinus of Valsalva aneurysm rupture (SOVAR) is an uncommon but serious cause of heart failure. Peripartum presentation is exceedingly rare and may mimic more common conditions such as peripartum cardiomyopathy. Early diagnosis is critical, as outcomes depend on prompt recognition and intervention.
Case Presentation:
A 30-year-old woman presented two months postpartum with progressive dyspnea, orthopnea, leg edema, and intermittent chest discomfort. Initial work-up revealed elevated BNP, biventricular enlargement, and moderately reduced left ventricular ejection fraction. Transthoracic and transesophageal echocardiography revealed a mobile structure adjacent to the tricuspid valve and severe right heart dilation. Cardiac magnetic resonance (CMR) imaging confirmed a ruptured noncoronary sinus of Valsalva aneurysm extending into the right atrium, with a significant left-to-right shunt (Qp:Qs 2.7). Computed tomography angiography further delineated the defect and ruled out coronary artery disease. The patient responded well to diuretic and guideline-directed medical therapy and was referred for surgical repair.
Discussion:
This case highlights the diagnostic challenges of postpartum dyspnea and underscores the importance of a multimodality imaging strategy. CMR enabled definitive diagnosis, volumetric analysis, and accurate shunt quantification, proving instrumental in differentiating structural heart disease from peripartum cardiomyopathy.
Conclusion:
Peripartum SOVAR is a rare but treatable cause of heart failure. Multimodal imaging—including echocardiography, CT angiography, and CMR—is essential to identify structural lesions, characterize shunt physiology, and guide management in complex cases.
A 30-year-old woman presented to the Emergency Department two months postpartum following the delivery of her third child, with progressively worsening dyspnea. Her symptoms had initially begun during the third trimester and were attributed to the physiological changes of pregnancy. However, the shortness of breath persisted and intensified in the postpartum period, occurring even with minimal exertion. She also reported occasional burning chest pain, orthopnea, abdominal distension, bilateral lower extremity edema, and intermittent presyncopal episodes.
Initial laboratory evaluation revealed a significantly elevated brain natriuretic peptide (BNP) level, while serial troponins remained within normal limits. Chest imaging demonstrated a markedly enlarged cardiac silhouette. Electrocardiography showed normal sinus rhythm, left atrial enlargement, a rightward QRS axis, and an incomplete right bundle branch block. Physical examination was notable for jugular venous distension, a cardiac murmur, and pulmonary rales.
Given the clinical presentation and initial findings, the patient was provisionally diagnosed with acute decompensated heart failure, with peripartum cardiomyopathy as the leading differential. Computed tomography angiography was performed to exclude pulmonary embolism, which was ruled out. However, the scan revealed signs of pulmonary hypertension, right ventricular strain, and abdominal ascites.
Transthoracic echocardiography demonstrated a moderately reduced left ventricular ejection fraction (EF 40–45%), a mobile structure adjacent to the tricuspid valve, severe tricuspid regurgitation, and dilation of the right-sided heart chambers. Coronary angiography revealed normal coronary anatomy and no significant coronary artery disease. Transesophageal echocardiography further identified a sinus of Valsalva aneurysm involving the noncoronary cusp. A cardiac magnetic resonance imaging (CMR) study was subsequently ordered to further evaluate cardiac structure and function.
Cardiac magnetic resonance imaging revealed asymmetric coronary sinuses, with a markedly dilated and ruptured non-coronary sinus of Valsalva aneurysm projecting into the right atrium, adjacent to the septal leaflet of the tricuspid valve (Figures 4-7). Continuous left-to-right shunting was visualized on both CINE and phase-contrast flow imaging sequences. There was no evidence of dissection or significant dilation involving the remaining thoracic aorta.
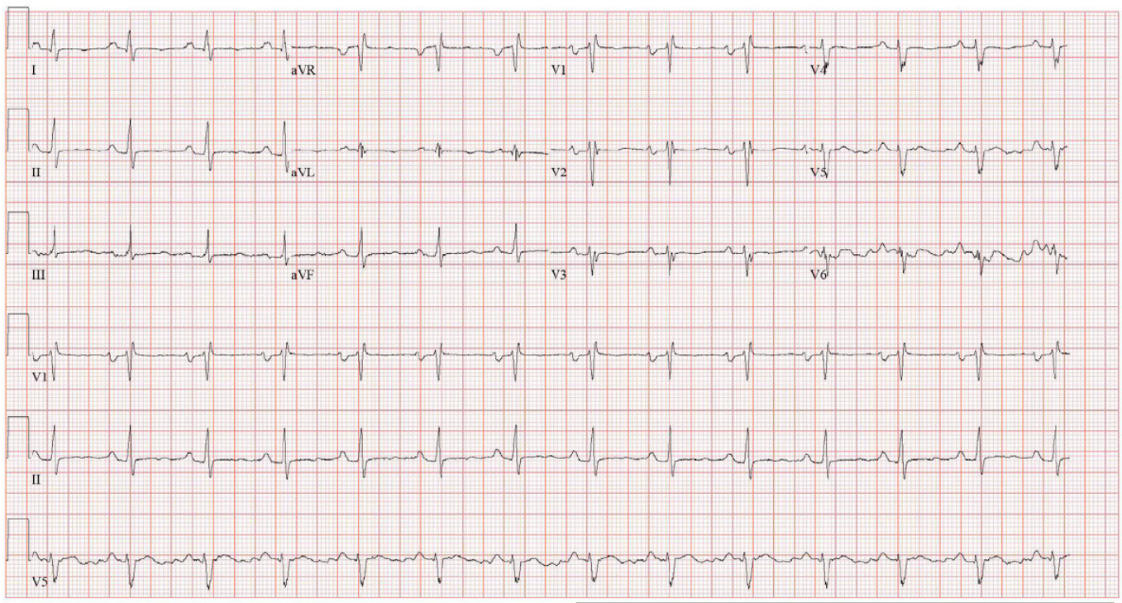
Figure 1: Electrocardiogram (ECG) demonstrating normal sinus rhythm with left atrial enlargement, rightward QRS axis, and incomplete right bundle branch block.
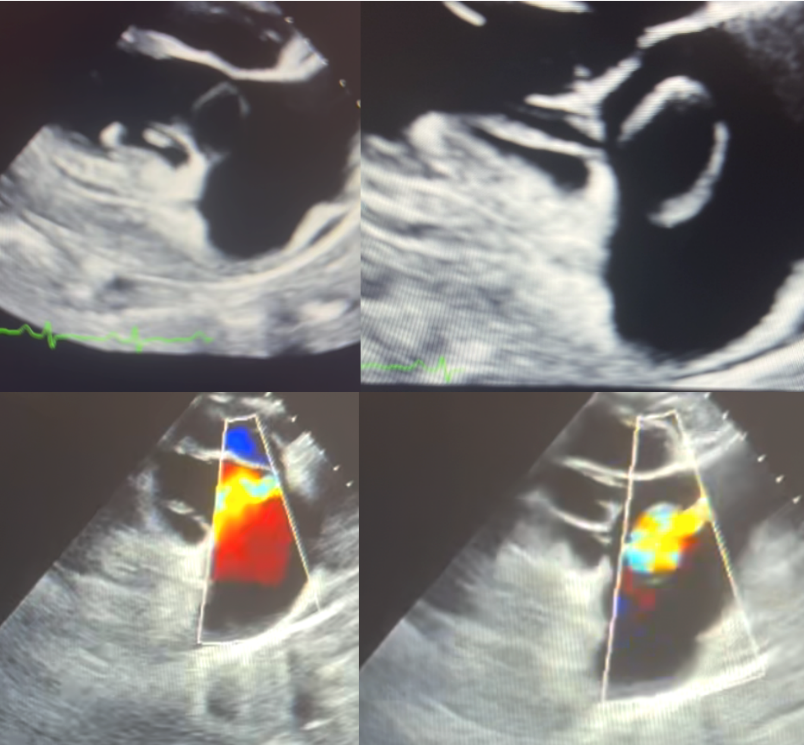
Figure 2: Transesophageal echocardiographic evaluation of the tricuspid valve. The left panes depict diastole, and the right panes depict systole; corresponding color Doppler imaging is shown in the lower panels. A cystic-appearing structure adjacent to the tricuspid valve annulus is noted, with color flow observed during both systole and diastole—findings suggestive of an aorto-atrial shunt from a ruptured sinus of Valsalva aneurysm.
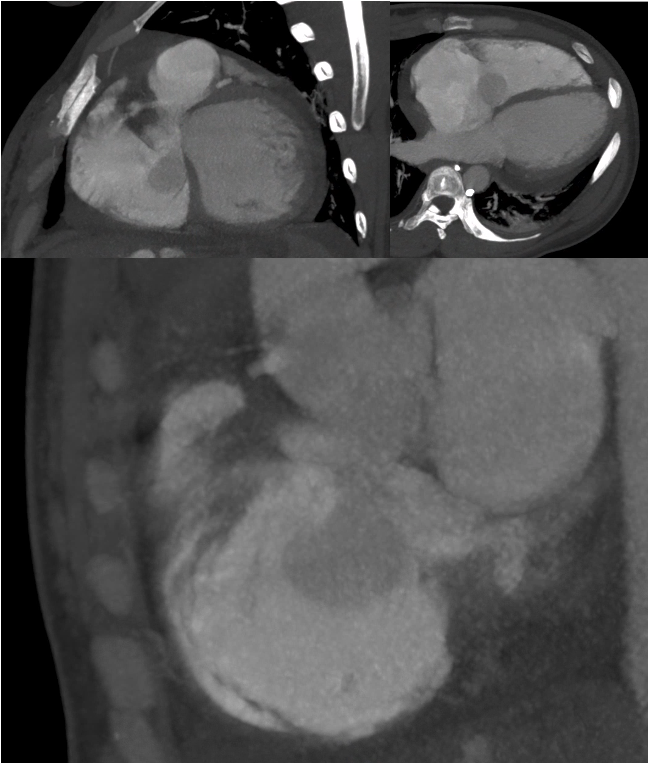
Figure 3: Nongated chest computed tomography angiography (CTA). Axial and coronal views demonstrate a prominent outpouching from the noncoronary sinus of Valsalva projecting into the right atrium, consistent with aneurysmal rupture. A region of swirling contrast with similar attenuation to the ascending aorta is visualized within the right atrium, indicative of a significant left-to-right shunt. Although limited by motion artifact and non-gated acquisition, the study provided key diagnostic clues supporting the diagnosis of ruptured sinus of Valsalva aneurysm (SOVAR).
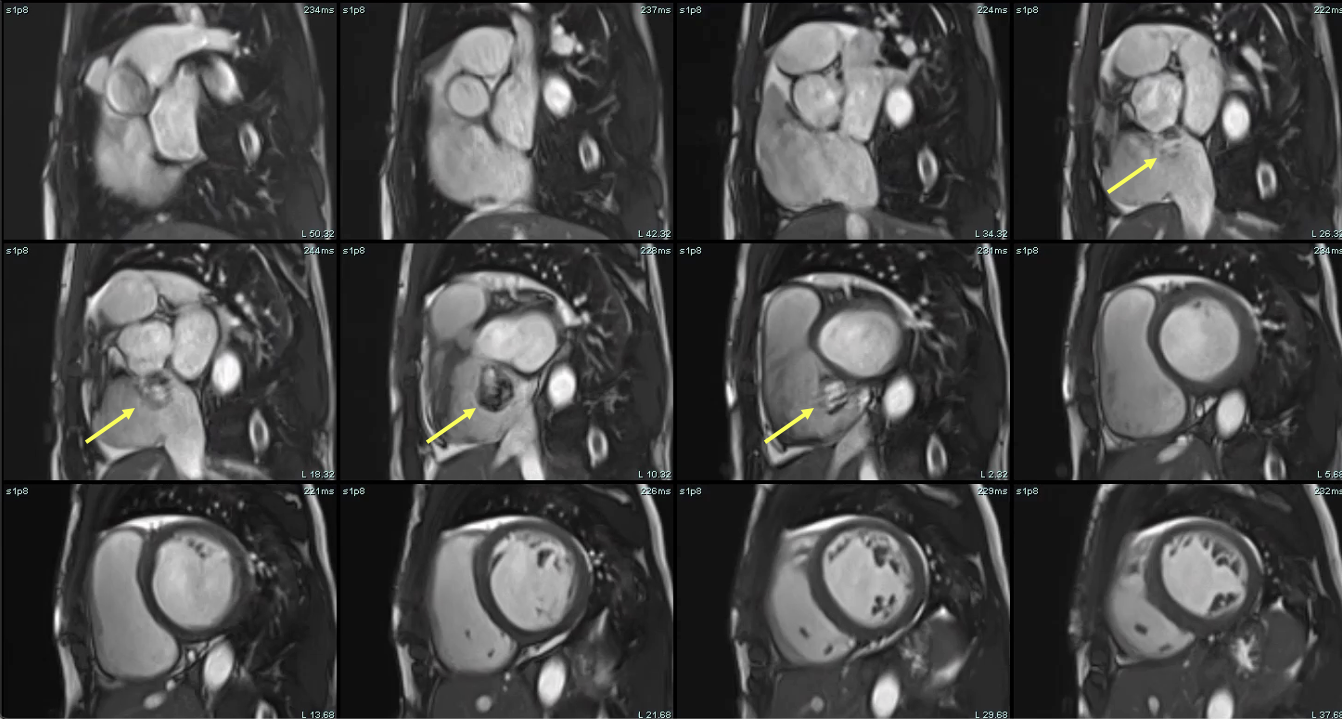
Figure 4: Cardiac magnetic resonance (CMR) short-axis steady-state free precession (SSFP) cine stack. Images demonstrate severe biventricular enlargement and a prominent cystic structure adjacent to the aortic root, consistent with a ruptured noncoronary sinus of Valsalva aneurysm projecting into the right atrium - yellow arrows highlight the site of rupture. The aneurysmal structure is contiguous with the aortic root and demonstrates dynamic filling, supporting the presence of an aorto-atrial communication.
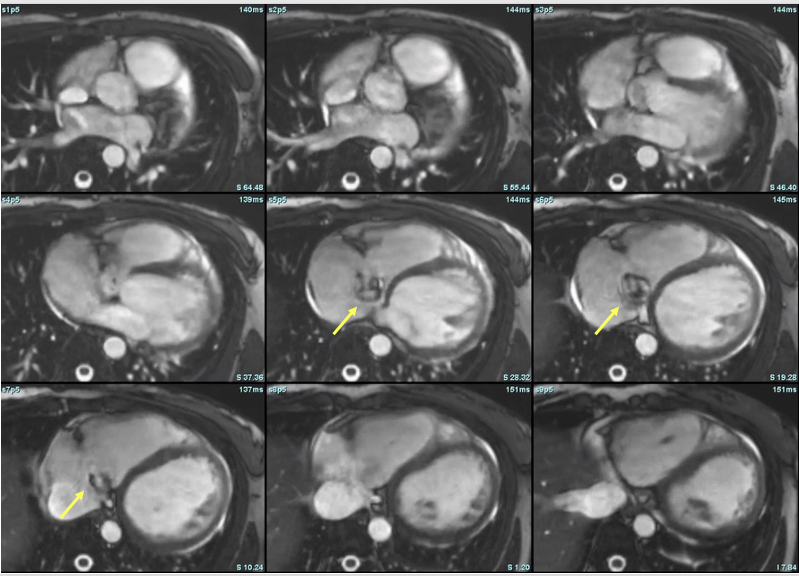
Figure 5: Axial steady-state free precession (SSFP) cine images from cardiac magnetic resonance (CMR), illustrating dynamic visualization of the ruptured sinus of Valsalva aneurysm. Yellow arrows denote the aneurysmal outpouching arising from the noncoronary sinus and extending into the right atrium. The signal variation and contour changes across frames reflect systolic-diastolic flow and deformation of the aneurysm, confirming an aorto-atrial communication. These images complement the short-axis stack in Figure 4 and emphasize the utility of multiplanar CMR imaging in localizing and characterizing shunt lesions.
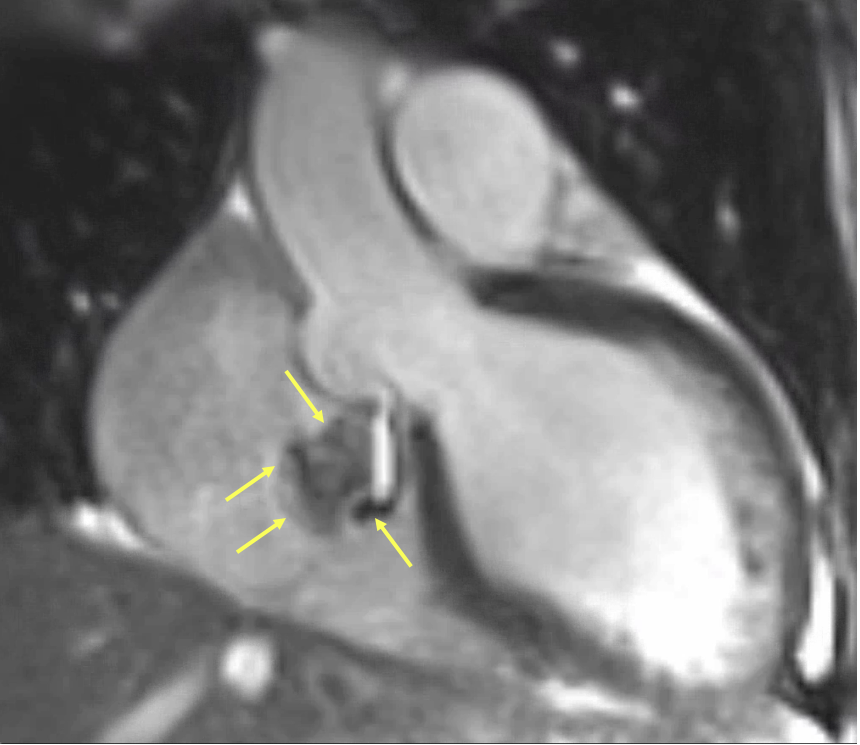
Figure 6: Magnified cardiac magnetic resonance (CMR) steady-state free precession (SSFP) cine still highlighting the ruptured sinus of Valsalva aneurysm. Yellow arrows delineate the borders of the aneurysmal sac arising from the noncoronary sinus and its direct extension into the right atrium. The swirling signal void within the aneurysm represents turbulent high-velocity shunting flow. This frame captures the anatomical and functional features of the rupture with high clarity, confirming an aorto-atrial communication and supporting the diagnosis suggested in Figures 4 and 5.
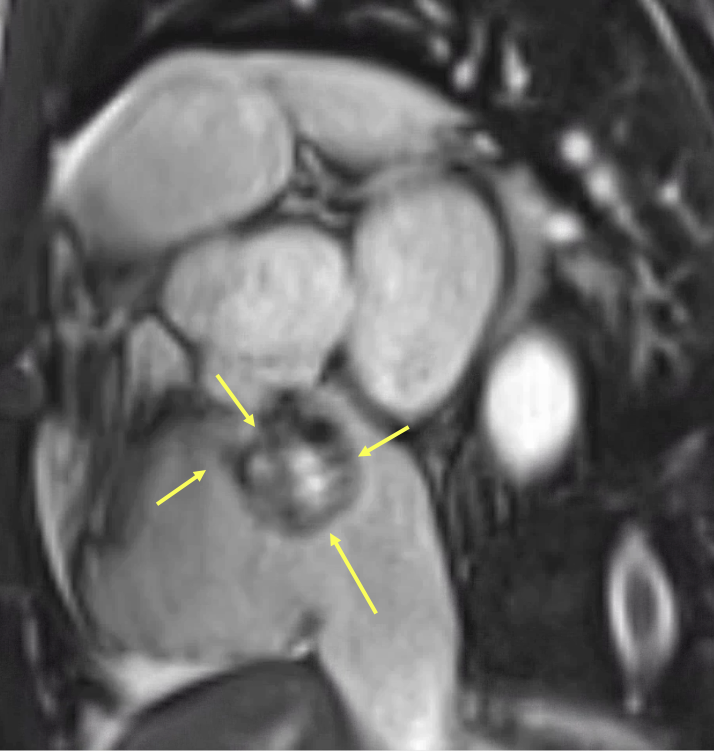
Figure 7: Cardiac magnetic resonance (CMR) short-axis steady-state free precession (SSFP) image at the level of the aortic root. Yellow arrows indicate the aneurysmal dilation arising from the noncoronary sinus of Valsalva, with a focal rupture site projecting into the right atrium. The central region demonstrates signal void consistent with turbulent high-velocity flow through the defect. This cross-sectional view confirms the origin and trajectory of the shunt, complementing the sagittal and axial projections shown in Figures 4–6.

Figure 8: Three-dimensional multiplanar reconstruction (3D MPR) from cardiac magnetic resonance imaging. Sequential static slices demonstrate an aneurysmal outpouching arising from the noncoronary sinus of Valsalva and projecting into the right atrium. The reconstruction highlights the anatomical morphology, orientation, and continuity of the rupture tract, offering precise localization of the aorto-atrial communication. This static dataset complements dynamic imaging by providing high spatial resolution for pre-surgical planning and anatomical delineation.
The pulmonary-to-systemic flow ratio (Qp:Qs), calculated using phase-contrast measurements at the ascending aorta and main pulmonary artery, was markedly elevated at 2.7–2.73 (Table 1), consistent with a significant left-to-right shunt. CMR also demonstrated severe biventricular chamber enlargement. Left ventricular ejection fraction was moderately reduced, while right ventricular ejection fraction was mildly reduced at 41% (Table 2). The tricuspid valve leaflets appeared structurally normal, with no evidence of stenosis. Mild tricuspid regurgitation was present, with a regurgitant fraction of approximately 8% on flow analysis. In contrast, moderate mitral regurgitation was observed, with a regurgitant fraction estimated at 38%, attributed to functional annular dilation.

Table 1: Biventricular volumetric and functional assessment by cardiac magnetic resonance imaging. The patient demonstrated severe biventricular chamber dilation with mildly to moderately reduced ejection fractions. Left ventricular (LV) and right ventricular (RV) cardiac outputs were markedly elevated, consistent with a high-output state likely driven by significant left-to-right shunting through the ruptured sinus of Valsalva aneurysm.

Table 2: Flow quantification by cardiac magnetic resonance (CMR) phase-contrast imaging. Main pulmonary artery (mPA) flow significantly exceeded systemic flow through the ascending aorta, consistent with a substantial left-to-right shunt. Systemic output was confirmed by summing flows through the superior vena cava (SVC) and descending aorta (dAo), yielding a Qp:Qs ratio of approximately 2.7.
We present a rare case of a peripartum ruptured sinus of Valsalva aneurysm in a patient who presented for the evaluation of progressive dyspnea, lower extremity edema, and concomitant occasional chest pain. Initial laboratory data, chest imaging, and physical examination all supported the diagnosis of acutely exacerbated heart failure with biventricular dilatation, pulmonary hypertension, and raising concerns for peripartum cardiomyopathy. CMR was pivotal in demonstrating the correct diagnosis of ruptured sinus of Valsalva with significant left to right shunt.
The patient was initiated on intravenous diuretics and guideline-directed medical therapy, including sacubitril-valsartan and dapagliflozin (Farxiga). In addition, iron supplementation was started for the treatment of anemia. She tolerated medical therapy well, with rapid improvement and eventual resolution of her symptoms. The patient remained hemodynamically and clinically stable, with complete resolution of her presenting symptoms and complaints. Cardiothoracic surgery was consulted for surgical evaluation.
Sinus of Valsalva aneurysm rupture into the right cardiac chambers is a rare congenital or acquired condition associated with significant morbidity and mortality (Weinreich et al., 2015). Its prevalence is estimated at 0.09% in the general population (Hope, 1842), most commonly involving the right coronary sinus, followed by the noncoronary sinus. The condition is more prevalent in men (4:1) and is significantly more common in individuals of Asian descent—up to five times higher than average—likely due to congenital predisposition (Feldman & Roman, 2006). Congenital causes include connective tissue disorders such as Marfan syndrome and Ehlers-Danlos syndrome. Acquired etiologies include trauma, atherosclerosis, infective endocarditis, iatrogenic injury, and syphilis. In our case, the aneurysmal rupture occurred in the peripartum period in a young woman without significant predisposing conditions—an extremely rare presentation with few documented cases in the literature (Huang et al., 2023; Latzman et al., 2006; Pamulapati et al., 1991).
The clinical presentation of SOVAR depends on several factors, including underlying comorbidities, the sinus involved, and the size of the defect. Acute rupture of a large aneurysm is associated with the worst prognosis, often presenting with sudden dyspnea, severe chest pain, and potential hemodynamic collapse. In contrast, a smaller, more gradual rupture may result in chronic, progressive symptoms that are more tolerable (Feldman & Roman, 2006). Despite its variable presentation, mortality remains high due to its elusive nature, underscoring the importance of a prompt, multimodal diagnostic approach when suspected.
A prominent sinus of Valsalva aneurysm rupture adjacent to the tricuspid valve annulus may precipitate significant tricuspid regurgitation in addition to creating an aorto-atrial shunt. This defect can lead to malcoaptation of the tricuspid leaflets due to wind-sock prolapse and forced systolic valve opening from the high-velocity shunting jet. While transthoracic echocardiography remains a cornerstone of initial cardiac imaging—particularly in patients presenting with heart failure symptoms—it may be limited in characterizing complex structural lesions. In such cases, a multimodality imaging approach is essential for accurately delineating the extent of the defect, identifying involved structures, cardiac chamber dimensions, assessing the severity of valvulopathy, and quantifying the degree of shunting. Different cases, such as in ours, may present with a cystic appearing lesion adjacent to the tricuspid valve demonstrating color flow. These parameters carry important diagnostic and prognostic value and are critical for guiding management.
Cardiac computed tomography angiography can also be a valuable tool in the diagnosis and management of sinus of Valsalva aneurysm rupture. This patient population is typically young and often at low to intermediate risk for obstructive coronary artery disease. In such cases, cCTA can reliably exclude coronary disease, potentially avoiding the need for invasive coronary angiography—particularly when the involved sinus corresponds to the origin of a coronary artery. Moreover, engagement of the coronary ostia during angiography may be technically challenging due to anatomical distortion and structural injury. While surgical repair remains the standard of care, emerging transcatheter, nonsurgical alternatives have been proposed (Latzman et al., 2006; Weinreich et al., 2015). In this context, cCTA can serve as an accurate tool for preprocedural planning. In our patient, SOVAR evaluation with chest CTA was partially limited by motion artifact at the level of the aortic root and suboptimal bolus timing for aortic evaluation. Nevertheless, it provided key diagnostic clues. Notably, there was evidence of negative contrast and swirling flow within the right atrium, largely isointense to the ascending aorta, consistent with a left-to-right shunt. Additionally, an aneurysmal outpouching of the non-coronary sinus projecting into the right atrium was clearly visualized.
Cardiac magnetic resonance imaging has been extensively validated for the evaluation of sinus of Valsalva aneurysm rupture. It enables precise quantification of cardiac chamber volumes, detailed assessment of valvular anatomy and function, and accurate measurement of flow parameters, including the pulmonary-to-systemic flow ratio (Qp:Qs). In addition to standard flow measurements at the aortic and pulmonary valves, CMR flow assessment can be cross-validated using alternative sites such as the descending aorta and superior vena cava. These secondary measurements provide internal consistency checks that enhance confidence in systemic and pulmonary flow quantification. In our case, systemic flow measurements obtained at the ascending aorta were concordant with those derived from the descending aorta and superior vena cava, thereby confirming the reliability of the findings and supporting the presence of a significant left-to-right shunt. The estimated Qp:Qs was approximately 2.7. Unfortunately, we were unable to obtain flow measurements at the left ventricular outflow tract or directly across the aortic valve. Such data would have enabled a more direct quantification of shunt volume and a more precise assessment of aortic regurgitation. This represents a learning opportunity for future cases involving similarly atypical presentations.
Furthermore, CINE imaging, particularly in non-standard planes tailored to individual anatomy, proved effective in visualizing diastolic flow into the receiving chamber, a key feature that helps distinguish shunt physiology from atrioventricular valve regurgitation. Additionally, the presence of sustained backward flow in the descending aorta has been previously correlated with significant aortic insufficiency. In our case, we postulate that this represents diastolic flow into the right atrium. This multimodal approach is especially valuable in evaluating complex structural lesions or in cases where initial echocardiographic findings are inconclusive. While right and left stroke volume measurements appeared relatively concordant, interpretation remains challenging. Nonetheless, the findings help exclude a pure intracardiac shunt such as a patent foramen ovale, atrial septal defect, or ventricular septal defect. However, these values must still be interpreted cautiously, given the volume overload imposed on one chamber and the potential presence of hemodynamically significant regurgitant valvular lesions.
An interesting finding in our patient’s case was the cystic appearance of the sinus of Valsalva aneurysm, which exhibited areas of signal loss on cardiac magnetic resonance imaging. Signal loss in CMR can arise due to several factors, including motion artifacts, flow effects, susceptibility artifacts, and intrinsic tissue properties. Given these potential causes, it is essential to tailor the interpretation to the specifics of each case. We hypothesize that signal loss is due to motion artifacts and turbulent blood flow, causing a void in the high-velocity shunting region. It is also important to note that certain pulse sequences and modifications in acquisition parameters can either exacerbate or mitigate signal loss. For instance, gradient echo sequences, while effective at minimizing some types of artifacts, are more susceptible to a loss of signal-to-noise ratio, making the choice of imaging parameters critical for accurate assessment and diagnosis. At times, a combination of different acquisition sequences and imaging parameters, rather than an either-or approach, should be considered to achieve a comprehensive and accurate evaluation.
It is crucial to exclude infective endocarditis as part of the differential diagnosis in patients presenting with sinus of Valsalva aneurysm rupture. First, infective endocarditis may serve as the underlying etiology, particularly in acquired cases where bacterial infection leads to focal destruction and weakening of the aortic wall or sinus. Second, its presence significantly increases the risk of morbidity and mortality due to complications such as septic embolization, abscess formation, valvular destruction, and systemic sepsis. The clinical presentation of SOVAR can closely resemble that of endocarditis-associated rupture, with both potentially manifesting as acute or progressive heart failure. However, infective endocarditis may also present with constitutional symptoms—such as fever, chills, malaise, and weight loss—which can provide important diagnostic clues. In our patient, there were no indications that the presentation was consistent with infective endocarditis, as she did not exhibit fever, chills, or other systemic signs of infection. Recognizing these features is critical, as the presence of infection would fundamentally alter both the urgency and nature of medical and surgical management. Prompt identification through blood cultures and transesophageal echocardiography is essential to guide appropriate therapy and improve outcomes.
In this case, the cardiac magnetic resonance was instrumental in achieving the correct diagnosis of a ruptured sinus of Valsalva aneurysm. While other imaging modalities, including transthoracic echocardiography and cardiac computed tomography angiography, accurately described the aneurysm, they were unable to fully characterize the ruptured component or qualitatively and quantitatively assess the degree of shunting. The importance of CMR in such cases cannot be overstated; it allows for precise chamber volume quantification, accurate dimensional evaluation, and is considered the gold standard for biventricular ejection fraction estimation. CMR also provides unparalleled shunt quantification, which is critical in evaluating the severity of left-to-right shunting. Additionally, the ability to obtain 3D imaging and CINE sequences offers valuable insights that are difficult, if not impossible, to capture through TTE or transesophageal echocardiography. Although cardiac CTA can play a role in diagnosing such conditions, its protocol is limited to imaging only one cardiac cycle or a portion of it, making it less effective in capturing dynamic aspects of aneurysms and shunts. Ultimately, CMR proved to be essential in diagnosing the ruptured sinus of Valsalva aneurysm and guiding the management and therapeutic decisions of the patient.
This case highlights a rare but life-threatening presentation of ruptured sinus of Valsalva aneurysm in the peripartum period, initially masquerading as peripartum cardiomyopathy. The diagnostic complexity underscores the need for a high index of suspicion and a systematic, multimodality imaging approach. Cardiac magnetic resonance imaging was instrumental in accurately identifying the rupture, quantifying the left-to-right shunt, and excluding other structural or ischemic etiologies. This case reinforces the critical role of advanced imaging in evaluating postpartum patients with unexplained dyspnea and emphasizes the importance of considering structural heart disease—even in the absence of classic risk factors or overt signs. Early diagnosis and tailored therapy can lead to favorable clinical outcomes, even in uncommon and deceptive presentations such as this.