AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/2690-4861/731
1Ege University, Faculty of Fisheries, Department of Marine–Inland Waters Sciences and Technology a, 35100, Bornova, İzmir, Turkey.
2Istanbul Technical University Development Foundation Schools, Nesan Campus, 35410, Menderes, İzmir, Türkiye.
*Corresponding Author: Özlem Çakal Arslan, Ege University, Faculty of Fisheries, Department of Marine–Inland Waters Sciences and Technology a, 35100, Bornova, İzmir, Turkey.
Citation: Özlem Ç. Arslan, Kaan Arslan, Başak Topçu, Koray Benas, (2025), Determination of Cancerogenic Potentials Microplastic and recycled plastic on Oreochromis niloticus using Micronucleus Assay, International Journal of Clinical Case Reports and Reviews, 24(4); DOI:10.31579/2690-4861/731
Copyright: © 2025, Özlem Çakal Arslan. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 28 February 2025 | Accepted: 13 March 2025 | Published: 25 March 2025
Keywords: micronucleus assay; cancerogenicity; genotoxicity; mikroplastics; nile tilapia
Micronuclei (MN) play a fundamental role during cancer evolution and metastatic progression. Water pollution means that these bodies are polluted with harmful substances, resulting in serious health hazards. Microplastics are currently a significant source of global concern. Environmental exposures are known risk factors for cancer etiology. These carcinogens also accumulate in aquatic organisms consumed by humans, eventually entering our food chain.
In our study, the effects of microplastic (MP) and recycled plastic (RP = plastic bottle caps) concentrations on the erythrocytes of the fish Oreochromis niloticus (Nile Tilapia) were investigated for their carcinogenic and hematological effects on red blood cells (RBCs) through MN testing and erythrocyte counting. The results indicated that, due to the effects of MP and RP concentrations, the frequency of MN in erythrocytes increased in a concentration- and time-dependent manner, leading to cancer in living organisms and causing a decrease in the number of red blood cells (anemia).
Aquatic ecosystems are deteriorating day by day due to various impacts and activities. These effects lead to a decrease in biodiversity, a decline in the quality of water resources, and the rapid disappearance of aquatic ecosystems. The aim of studies on the health of ecosystems is to investigate the effects of pollution on living organisms, to reveal the damage caused by pollution, to seek solutions for reducing this damage, and to collect data to take necessary precautions before populations become extinct in the future. Recently, one of the groups that has caused pollution in aquatic ecosystems to reach dangerous levels and poses a significant risk is micro-plastic pollution. Microplastics are any synthetic solid particles or polymeric matrices that are insoluble in water, ranging from 1 µm to 5 mm, and can be either regularly or irregularly shaped. It is indeed believed that this new definition is explanatory in every respect and will be useful for all microplastic monitoring and comparison studies conducted worldwide (Yurtsever, 2018). They are classified as primary and secondary microplastics (MPs) based on their production forms (Li et al., 2018). Primary MPs are small plastic particles that are primarily found in pharmaceuticals, textiles, and personal care products, and are released directly into the aquatic environment (Cole et al., 2011; Browne, 2015). Primary MPs can be transported into aquatic environments (Gall and Thompson, 2015). Secondary MPs are formed due to the gradual breakdown of larger plastic particles present in the ecosystem (Arthur et al., 2009). They originate from fishing nets, household items, resin materials, and single-use plastic products (Eerkes-Medrano et al., 2015). Today, MPs are the most abundant non-depleting type of pollutant found on Earth (Derraik, 2002; Galgani et al., 2013). MPs raise public concern due to their ubiquitous presence in aquatic environments (Li et al., 2018). The entry of microplastics into aquatic environments primarily occurs through rivers, lakes, and oceans. The transportation of these materials by rainwater surface runoff or the direct discharge of waste into aquatic environments contributes to microplastic pollution. Many aquatic organisms consume these small particles, mistaking them for food. As a result, microplastics become part of the food chain and threaten the health of organisms in the ecosystem. Microplastics pose a danger not only to animals but also to humans. Microplastics that enter the human body through seafood carry potential health risks. Current research suggests a potential link between these ubiquitous plastic particles and cancer, shaking the foundations of our understanding of health risks associated with plastic pollution. Studies are investigating the profound effects of microplastics on human health, particularly their roles in cancer development and progression, and have begun to reveal a disturbing relationship between exposure to microplastics and increasing cancer cases, utilizing comprehensive data analysis and laboratory experiments (Park et al., 2023; Li et al., 2023). One of the most concerning findings of these studies is the ability of microplastics to act as carriers of carcinogenic compounds (Rahman et al., 2021). These plastic particles, generally invisible to the naked eye, have a remarkable capacity to absorb and accumulate toxic chemicals from the surrounding environment. When ingested or inhaled, these microplastics can release these harmful substances directly into the body and may cause carcinogenic transformations in cells (Chen et al., 2020). Furthermore, the ubiquitous presence of microplastics in various ecosystems poses a threat to human health (Xu et al., 2024).
Cancer is increasingly recognized as a genomic disease characterized by the accumulation of genetic damage. This genetic alteration is often reflected in the formation of micronuclei (MN), which are small nuclei that occur due to chromosomal fragmentations and errors in cell division. Recent research underscores the significance of MN as a potential early diagnostic marker in cancer patients, indicating its fundamental role in tumor evolution and metastatic progression (Di Bona et al., 2024; Boveri, 2008). One pressing public health issue closely related to cancer risk is water pollution, which encompasses the contamination of groundwater and drinking water with various harmful substances. Such environmental pollutants, as highlighted by Garcia and Matthews (2024), pose serious health hazards, particularly concerning their established link to cancer development. Numerous studies have shown that carcinogens present in contaminated water sources significantly elevate the risk of various cancer types. These toxins can bioaccumulate in aquatic organisms, making their way into the human food chain and thus amplifying the exposure risk for consumers (Garcia and Matthews, 2024). As environmental exposures are recognized as key risk factors in cancer etiology, biomarker-based approaches have emerged as valuable tools for assessing individual and population-level exposure to genotoxic and carcinogenic agents (Boveri, 2008). In this context, the MN test stands out as a well-established biomonitoring method used to evaluate DNA damage at the chromosomal level. It provides critical insights into the genomic instability characterizing cancerous tissues and facilitates early detection of disease-related changes (Arslan et al., 2015; Arslan and Parlak, 2016, 2017).
Freshwaters are a source, carrier medium, and habitat for microplastics (MPs) (Klein et al., 2018). Freshwaters can accumulate a significant amount of MPs; however, there have been very few studies comparing MPs in freshwater to those in seawater (Li et al., 2018). For example, MPs can cause neurological dysfunction in rock gobies (Pomatoschistus microps) (Oliveira et al., 2013) and increase mortality in European sea bass (Dicentrarchus labrax) (Mazurais et al., 2015). Fish-based biomarkers are important for monitoring pollution stress and ecosystem changes. Biochemical parameters are considered useful bioindicators for assessing the effects of various pollutants in fish (Osman et al., 2018; Sayed and Soliman, 2018). In recent years, the use of hematological parameters as a significant tool for environmental pollution has been emphasized (Sayed and Authman, 2018; Fazio, 2019). Monitoring changes in biochemical parameters can provide early warning indicators of general and specific toxicological responses. Biochemical biomarkers of pollution are important indices used in fish toxicity tests and field monitoring of water pollution. In toxicological studies of exposure, increases in micronucleus frequency may reflect cell damage in specific organs and are also known as a biomarker of pollution-induced carcinogenesis in fish. Fish are widely used to assess the health of aquatic systems and their biological responses serve as biomarkers of environmental pollutants.
Nile Tilapia (Oreochromis niloticus) is considered to be the most important wild and cultured freshwater fish in Africa (Soliman, 2017). In addition, Nile Tilapia has been used in toxicological studies (Osman et al., 2018). Accordingly, the present study aimed to investigate the effects of MPs on the hematological parameters of early juveniles of popular fish (Oreochromis niloticus). In our study, the effects of Microplastic and Recycled Microplastic were also determined as hematological parameters such as red blood cell count (RBCs). Nile tilapia is one of the most common freshwater fish used in toxicological studies (Almeida et al., 2001), as it presents a set of characteristics that could make it a suitable model to be used as an indicator species in biomonitoring programs (Mohamed et al., 2023). Biomarker studies in water bodies in many countries have been extensively documented, but scientific reports on the carcinogenic effects of microplastics and recycled plastic are lacking.
Fish (O. niloticus) were obtained from the Aquaculture Department, Aquarium Unit, Faculty of Fisheries, Ege University. The average weight of the fish was 6.06 ± 1.40 g and the average length was 7.56 ± 0.80 cm. The fish were subjected to adaptation (acclimatization) for 2 weeks under laboratory conditions in a large tank (pH: 7.85; alkalinity: 154 mg/L CaCO3; DO: 6.70 mg/L; temperature: 22 °C) before the experiment. The photoperiod used was 12/12 h dark/light. The aquariums used in our study were made of glass, provided continuous ventilation, had physical dimensions of 100 × 20 × 20 cm and a capacity of 10 L.In the experiments, 1 mm transparent microplastics and blue recycled microplastics obtained from plastic bottle caps were used. The microplastics were weighed directly and placed in aquariums containing 10 liters of water at concentrations of 0.1, 10 and 25 mg/L.After the end of acclimation, 10 fish in each aquarium were tested. Fish were divided into 8 groups, each containing 1 fish. Group 1: Negative, control (only tap water was used); Group 2: Positive control (10.0 mg/CdCl2; Sigma); Group 3: 1 mg/L; and Group 4: 10.0 mg/L, Group 5: 25 mg/L Clear Microplastic- Group 6: 1 mg/L, Group 7: 10 mg/L and Group 8: 25 mg/L Blue recycled Microplastic concentrations were selected according to literature data (Almeida et al., 2001).
Fish (O. niloticus) Micronucleus Test
Peripheral blood samples were collected from the tail vein of fish on the 4th day of the exposure period. A smear was made by placing a drop of blood on a slide. 10 fish were used for each group and at each interval, and the blood was immediately processed for MN tests. In each evaluation, 1000 cells/fish were analyzed, 10.000 erythrocytes for each group. Blood samples were placed on 3 clean microscopic slides for each fish, fixed in pure ethanol for 10 min, and allowed to dry at room temperature. Then, 10% Giemsa (w/v) staining was performed for 10 min. In each preparation, 1000 erythrocytes were examined under a light microscope using 1000x magnification to determine the frequencies of micronuclei and nuclear abnormalities. For the detection of micronuclei, the criteria of Al-Sabti and Metcalfe (1995) were taken into account: MN should be smaller than one third of the main nuclei and should be clearly separated from the main nucleus.The occurrence and frequency of MN/BN were counted as 1000 cells from each living organism and the frequency of MN/BN (Figure 1) was calculated as ‰ (Bolognesi and Fenech, 2012).
Hematological parameters
Hematological parameters RBCs (-red blood cell count) were performed with Neuber blood counting chamber (Mohamed et al., 2019)
Data from treatments and controls were compared using one-way analysis of variance (ANOVA), followed by Dunnett’s comparison post-hoc test whenever applicable (p ˂ 0.05) to compare the mean differences in MN frequency between groups and exposure durations. Results with P < 0>
In our study conducted with peripheral erythrocytes (blood cells) of O. niloticus exposed to 1, 10 and 25 mg/L Microplastic and Recycled Plastic concentrations for 4 days, two types of nuclear abnormalities were detected. Micronucleated (MN) and Binucleated (BN) cells. Abnormalities in the structure of the nucleus such as lobed and divided were also observed, but they were not included in the calculations because they were few in number (Figure 1) (Table 1).
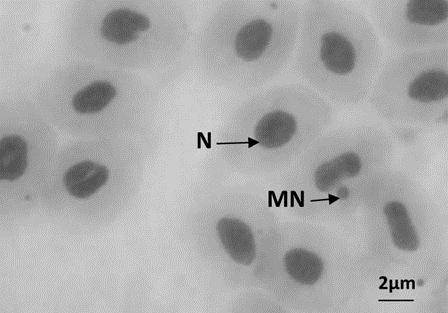
Figure 1: a: Erythrocytes with normal nuclei (magnifying power: 1000×), b: Erythrocytes with micronucleus (MN)
In the peripheral erythrocytes of O. niloticus exposed to 1, 10 and 25 mg/L Microplastic and Recycled Plastic concentrations for 96 h, a high increase in the frequency of Micronuclei (Figure 1, 2), which are early markers of cancer, was observed due to the increase in MP and GP concentrations compared to the control group. In the positive control group, Cadmium (CdC2), a high rate of MN was detected. Micronucleus and Binucleus frequencies are given in Table 1.
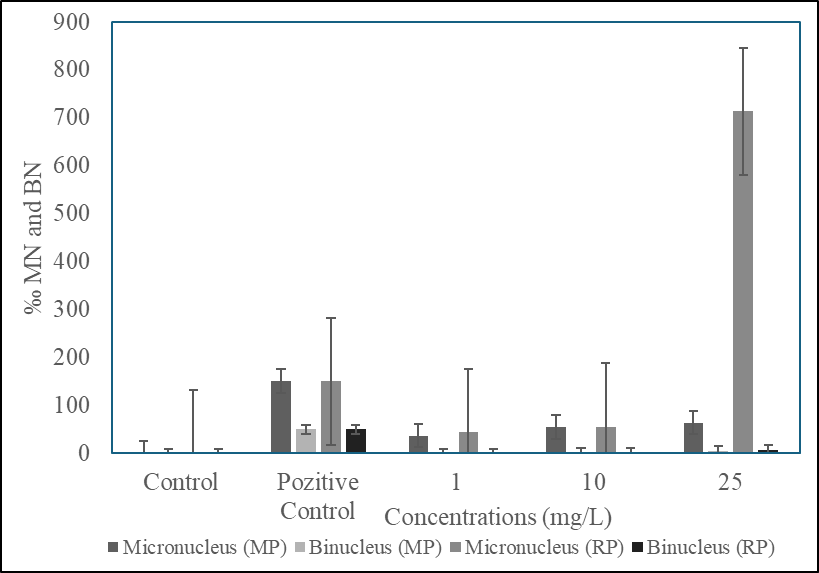
Figure 2: Micronucleus frequencies in erytrocytes depending on exposure time (96h)
| Microplastik | Nucleus | Micronukleus (n=1000) | Binucleus (n=1000) |
| Control | 1000±0 | 0±0 | 0±0 |
| Pozitive Control (Cdcl2) | 800±1,74 | 150±3,33 | 50±7,09 |
| 1 mg/L | 963,2±4,19 | 36,90±12,67 | 0±0 |
| 10 mg/L | 944±7,74 | 55,00±1,33 | 1,0±1,0 |
| 25 mg/L | 932±2,4 | 64±4,01 | 4,3±0,2 |
| Recycled Plastic | |||
| 1 mg/L | 957,2±1,9 | 43,90±2,6 | 0±0 |
| 10 mg/L | 942±8,5 | 57,0±3,03 | 2,0±1,0 |
| 25 mg/L | 922±2,4 | 714±4,01 | 6,9±2,2 |
Table 1: Nucleus and Micronucleus score of erythrocytes (‰)
It was observed that the micronucleus frequencies in both the MP and GP doses and the positive control group were significantly higher (P < 0>
Empty Cell (Microplastic) | Exposure Time (96 h) | |||
| Control | 1 mg/L MPs | 10 mg/L MPs | 25 mg/L MPs | |
| (RBCs) (Milyon/mm3) | 1.9 ± 0.02a | 1.52 ± 0.01b | 1.5 ± 0.01b | 1.3 ± 0.01c |
Empty Cell (Recycled Plastic) | Exposure period (96 h) | |||
| Kontrol | 1 mg/L GPs | 10 mg/L GPs | 25 mg/L of GPs | |
| (RBCs) (Milyon/mm3) | 1.9 ± 0.02a | 1.42 ± 0.01b | 1.31 ± 0.01b | 0,8 ± 0.02c |
Table 2. Effect of 4 days (MP and GP) exposure on hematological parameters of tilapia (Oreochromis niloticus) early juvenile fish.
In environmental mutagenesis, MN tests provide quite practical results in monitoring the clastogenic and genotoxic effects of pollutants. Fish are primary indicators of the health of aquatic environments. To obtain these results, aquatic organisms such as as fish like rainbow trout Oncorhynchus mykiss and Oreochromis niloticus are commonly used (Cavaş and Ergene 2003, Tsarpali and Dailianis 2012). Previous studies have examined aquatic organisms exposed to polluted waters to determine the effects of genotoxins in natural environments (Barśiene and Barśyte 2000; Dixon et al. 2002). The use of the micronucleus test to determine the genotoxic status of aquatic environments is rapidly increasing (Arslan et al. 2015, Dailianis et al. 2003, Tsarpali and Dailianis 2012).
Cadmium has been found to significantly trigger micronucleus formation in various tissues of Oreochromis mossambicus (Chandra and Khuda-Bukhsh, 2004), Salmo trutta, and Phoxinus phoxinus (Sanchez-Galan et al., 1999). Previous studies have indicated that chemicals cause nuclear abnormalities in various tissues of fish (Palheres and Grisolia, 2002; Talapatra Banerjee, 2007). There is a paucity of information on the effects of microplastics (MP) in early juvenile stages of freshwater fish. The present study aimed to investigate the accumulation of microplastics (MP) and exposure on blood biomarkers in early juvenile stages of Nile Tilapia (O. niloticus). Nile Tilapia is considered to be the most important wild and cultured freshwater fish in Africa (Soliman, 2017). In addition, Nile Tilapia is frequently used in toxicological studies (Osman et al., 2018). In environmental mutagenesis, MN tests provide very practical results in monitoring the clastogenic and genotoxic effects of pollutants. Fish and mussels are the main indicators of the health of the aquatic environment. To obtain these results, aquatic organisms such as bivalves Mytilus galloprovincialis, Crassotrea gigas and Chamelea galina and fish rainbow trout Oncorhynchus mykiss, Oreochromis niloticus (Cavaş and Ergene 2003, Arslan et al. 2010, Tsarpali and Dailianis 2012) are generally used. In previous studies, aquatic organisms exposed to polluted waters were examined to determine the effects of genotoxins in their natural habitats (Barśiene and Barśyte 2000; Dixon et al. 2002). The use of micronucleus test in determining the genotoxic status of aquatic environments is rapidly increasing (Arslan et al. 2010, Dailianis et al. 2003, Tsarpali and Dailianis 2012). The results of our study showed that micronuclei were formed in the peripheral erythrocytes of O. niloticus when exposed to different concentrations of MP and GP and that their high frequency caused cancer. Consistent with our findings, previous studies reported that micronucleus tests in fish erythrocytes and other tissue cells gave positive results after cadmium application. Cadmium induced significant micronucleus formation in different tissues of Oreochromis mossambicus (Chandra and Khuda-Bukhsh, 2004), Salmo trutta and Phoxinus phoxinus (Sanchez-Galan et al., 1999). Similarly, Çavaş et al. (2005) reported increased MN induction in peripheral blood erythrocytes, gill epithelial cells and liver cells of Cyprinus carpio, Carassius gibelio and Corydoras paleatus with exposure to Cd doses. Previous studies have reported that chemicals cause nuclear abnormalities in different tissues of fish (Palheres and Grisolia, 2002Talapatra Banerjee, 2007). In the present study, significantly higher numbers of MN were observed in groups exposed to microplastics compared to the control group. In line with the present result, Lu et al. (2016) reported significant amounts of Microplastics in different organs of zebrafish. Furthermore, MPs were bioaccumulated in different organs of male mice (Mus musculus) (Deng et al., 2017). Similarly, Pitt et al. (2018) observed that microplastics accumulated in the yolk sac of zebrafish and migrated to different organs during embryogenesis. Furthermore, polystyrene MPs were reported to be absorbed from the intestinal mucosa and transported to different tissues of mussels by the circulatory system (Browne et al., 2008). In addition, it has been shown by researchers that the bioaccumulation of MP in (Oreochromis niloticus) increases depending on the dose and time (Ding et al., 2018). Hematological parameters are important measures reflecting the health status of fish (Thummabancha et al., 2016). The resulting anemia can cause suppression of red blood cells due to hematopoietic tissue damage (Wintrobe, 1978). Numerous studies have shown that the peripheral red blood cells, Hb and Hct of fish decrease after exposure to different pollutants under laboratory conditions (Osman et al., 2018). MP toxicity causes blood thinning due to the decrease in red blood cells and tissue damage (Tort et al., 1987). The deterioration in hematological parameters is interpreted as a defense response against MP toxicity (Osman et al., 2018). In addition, Espinosa et al. (2017) indicated that MP intake may alter the immune system with an effect on animal health and defense. The immune system may be affected by the chemicals that MPs may contain, absorb or release (which may be toxic (Rochman et al., 2013)) or by physically blocking digestive organs, reducing nutrient absorption and causing impaired energy distribution (Cole et al., 2011). Similarly, Ololade and Oginni (2010) observed a decrease in WBC count in Nile tilapia and African catfish after nickel exposure. The decrease in white blood cells may be due to bioaccumulation of pollutants including MPs in the tissues.
The present study is consistent with previous studies showing that microplastics and recycled plastics are genotoxic/carcinogenic, dilute blood by causing a decrease in red blood cells and consequently anemia, and that genotoxins are mainly responsible for these nuclear changes.
Hu et al. (2023) reported significant accumulation of MPs in the gills, intestines, and liver of O. niloticus with microplastics. It was concluded that these results are due to the organisms accidentally consuming MPs as food due to their small size (Huang et al., 2020). A large number of chemicals can interfere with DNA synthesis in an exposed organism, resulting in nuclear abnormalities (Ventura et al., 2008). In our study, MN frequencies in RBCs increased with dose. Das and Nanda (1986) reported a dose- and time-dependent increase in the induction of micronuclei in the peripheral blood of Heteropneustes fossilis. Yadav and Trivedi (2009) found that micronucleus frequencies increased in Channa punctata 96 h after heavy metal exposure, but the frequencies gradually decreased with continued exposure. Nepomuceno et al. (1997) suggested that higher concentrations of pollutants may inhibit normal cell division, damage RBC chromosomes, and disrupt DNA replication, resulting in reduced or severe micronucleus frequencies. In conclusion, the results of this study showed that genotoxic damage, revealed by micronucleus and nuclear abnormalities tests, increased in fish exposed to microplastics. The use of fish as biomarkers of pollution effects is becoming increasingly important and allows for early detection of aquatic environmental pollution. These organisms respond to toxic agents in a manner similar to higher vertebrates, which may allow for the assessment of substances that are potentially hazardous to humans. Therefore, it is imperative to determine the genotoxic effects of pollutants such as microplastics. The widespread presence of microplastics in the environment is a growing concern for public health, especially in relation to cancer. Evidence suggests a potential link between these small plastic particles and the development of cancer, as shown in experimental studies. Microplastics have been found to cause oxidative stress, inflammation, and cellular damage, all of which can lead to cancer. Public awareness and scientific progress are crucial to address this emerging threat and ensure a safer future for future generations.
As bioindicators, fishs exhibit responses to toxic agents that are analogous to those observed in higher vertebrates, thereby facilitating the assessment of potentially hazardous substances for human health. Consequently, it becomes imperative to elucidate the genotoxic effects of pollutants such as benzylparaben, in light of their pervasive presence and implications for public health, particularly concerning cancer risks.
It is seen that the MN test, which is used to determine the genotoxic effect of various chemicals with different species as ealy warning signals of cancer, is a safe test method. The limited number of studies on Microplastics and recycled plastics has been determined to cause a genotoxic effect, raises concerns. Therefore, more and different studies are needed in terms of the safety and sustainability of the aquatic ecosystem.