AUCTORES
Globalize your Research
Mini Review | DOI: https://doi.org/10.31579/2641-0419/474
1Specialist in Comprehensive General Medicine.
2Specialist in Non-Invasive Cardiology.
3Special Collaborator.
*Corresponding Author: Rachel Fernández Bravo, Specialist in Comprehensive General Medicine.
Citation: Rachel F. Bravo, Camilo F. Bravo, Odalys B. Rojas, (2025), Anatomic and Physiologic Surgical Strategies for Congenitally Corrected Transposition of the Great Arteries: A Comprehensive Review and Clinical Insights, J Clinical Cardiology and Cardiovascular Interventions, 8(7); DOI: 10.31579/2641-0419/474
Copyright: © 2025, Rachel Fernández Bravo. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 25 March 2025 | Accepted: 25 April 2025 | Published: 02 May 2025
Keywords: left ventricular non-compaction; cardiomyopathy; coronary artery disease; coronary artery bypass graft surgery
Left ventricular non-compaction cardiomyopathy, a rare congenital cardiomyopathy, is characterized by prominent trabeculations and deep intertrabecular recesses that communicate with the ventricular cavity. The typical presentation includes a triad of heart failure, ventricular arrhythmia, and systemic embolization. The disorder is often diagnosed by echocardiography and cardiac magnetic resonance imaging. In the absence of specific therapy, the management depends on the clinical presentation. Here, we describe a young man with left ventricular non-compaction cardiomyopathy and coronary artery disease who underwent coronary artery bypass graft surgery.
Left ventricular non-compaction cardiomyopathy (LVNC), also known as ‘Unclassified Cardiomyopathy’ by the World Health Organization or ‘Genetic Cardiomyopathy” by the American Heart Association, is a rare congenital disorder in the absence of any coexisting congenital lesion. In these patients, regression of the embryogenic sinusoids is impaired during ontogenesis by ventricular pressure overload that results in deep recesses that communicate with both the ventricular cavity and the coronary artery system. The reported prevalence of this disorder is about 0.01-0.26% in echocardiographic studies.1 The classical triad of heart failure (HF), arrhythmia, and systemic embolization is seen in most patients. LVNC is often diagnosed by echocardiography and cardiac magnetic resonance imaging (MRI). In this case report, we describe a patient with LVNC who had concomitant coronary artery disease (CAD).
A 38-year-old man with unstable angina and worsening dyspnea (New York Heart Association [NYHA] class IV) was admitted for evaluation. There was a history of frequent hospitalizations for HF. The electrocardiogram was suggestive of old anteroseptal myocardial infarction and left bundle branch block. Two-dimensional echocardiography revealed severe global hypokinesia of the LV with an ejection fraction (EF) of 15-20%. In addition, multiple coarse trabeculations were seen at the LV apex, suggestive of LVNC cardiomyopathy. His N-terminal proBNP level was 2600 pg/ml. Preoperative medications included heparin boluses, torsemide, atorvastatin, metoprolol succinate, ivabradine, and a combination of sacubitril and valsartan. Coronary angiography showed 80% stenosis of the left main coronary artery and 90% stenosis of the right coronary artery (Figure 1). Given the symptoms and coronary angiography findings, the patient was advised coronary artery bypass graft (CABG) surgery.

Figure 1: Coronary angiography images showing critical stenosis of left main coronary artery (Figure 1A) and right coronary artery (Figure 1B)
Under standard cardiac monitoring including transesophageal echocardiography (TEE) and pulmonary artery catheterization, a triple-vessel off-pump CABG was performed with intra-aortic balloon pump support. Intraoperative TEE confirmed the preoperative echocardiographic findings (Video 1). There were multiple, trabeculations and recesses with color flow at the LV apex (Figure 2). Poorly developed posterior papillary muscle was also seen. The calculated LVEF was 16.9%, and the left atrium (4.5 cm) and LV (6.7 cm end-diastole) were enlarged (Figure 3). The surgical procedure and the postoperative course were unremarkable. Before discharge from the hospital, the patient underwent cardiac resynchronization therapy with an implantable cardioverter defibrillator. An oral anticoagulant (acenocoumarol) was started to prevent thrombus formation in the LV. At three months follow-up, the patient had NYHA class II symptoms and the echocardiography showed an increase in LVEF to 30%
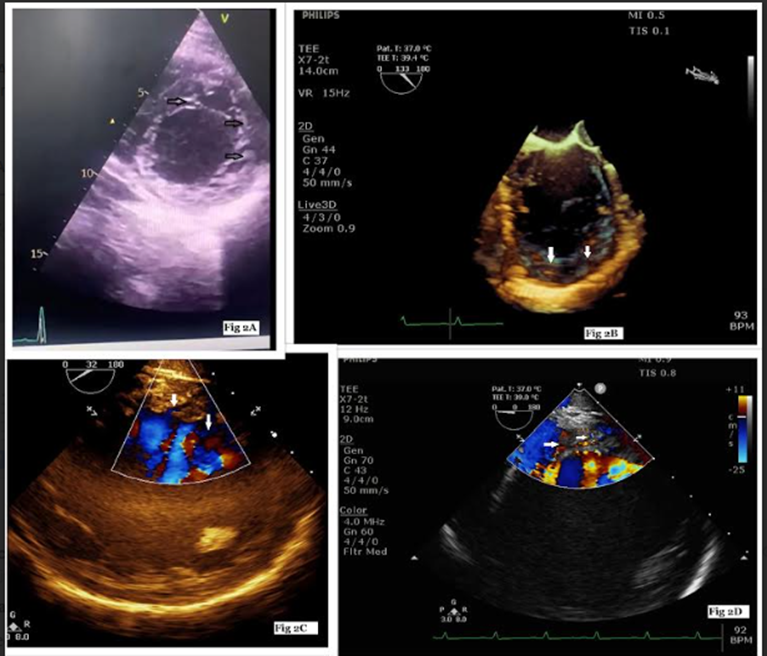
Figure 2: Apical short-axis view of the left ventricle on echocardiography showing prominent trabeculations and intertrabecular recesses at inferior and lateral walls (Figure 2A), live 3-D image (Figure 2B), inter-trabecular recesses filled by blood on color Doppler (Figure 2C, 2D).
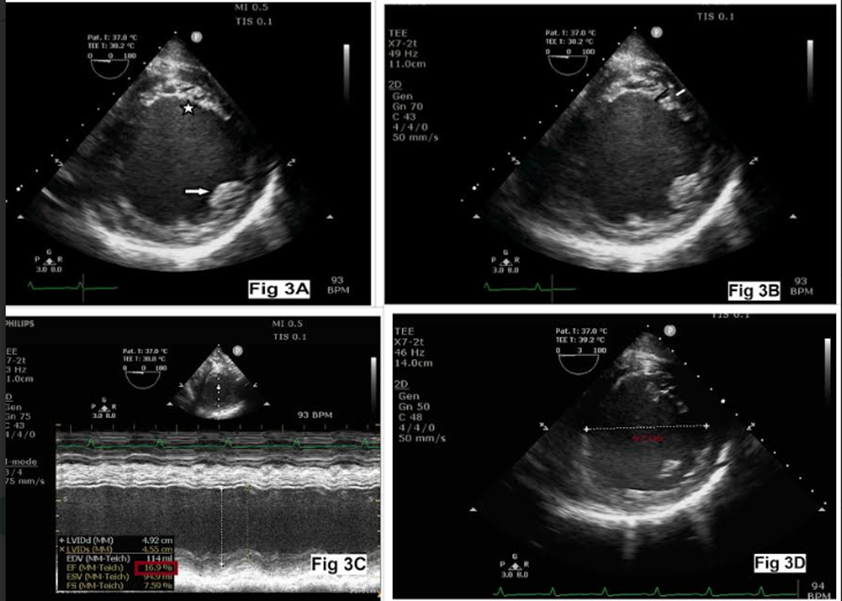
Figure 3: Normal anterior papillary muscle (arrow) with poorly developed posterior papillary muscle (star, Fig 3A), thick non-compact layer (black) and thin compact layer (white, Figure 3B), left ventricular ejection fraction 16.9% (Figure 3C), and dilated left ventricle (Figure 3D)
LVNC is a rare congenital cardiomyopathy, characterized by prominent LV trabeculations and deep intertrabecular recesses that communicate with the LV cavity. The reported prevalence of LVNC is about 0.01-0.26% in adults undergoing echocardiography.1 The myocardial wall in LVNC is composed of a thin, compacted (C) epicardial layer and a thicker, noncompact (NC) endocardial layer. The clinical presentation can vary from asymptomatic to symptoms of congestive HF, arrhythmia, embolization, and sudden death. However, the classical triad of HF, ventricular arrhythmia, and systemic embolization described in the literature, was not observed in this patient. Only a history of frequent hospitalizations for HF was present.
Dusek et al were the first to describe LVNC as persistent spongy myocardium with the embryonic blood supply in autopsy of children in 1975.2 Engberding and Bender made the antemortem diagnosis of LVNC in an adult using echocardiography, which demonstrated a spongy myocardium with prominent sinusoids; they attributed these findings to a lack of sinusoidal regression during cardiac embryogenesis.3 During embryonic life, before the coronary vessels develop, the intertrabecular recesses, or sinusoids, communicate with the LV cavity to receive blood supply. After the coronary vasculature develops, the ventricular myocardium gradually becomes compacted and the larger intertrabecular recesses are transformed into capillaries. However, in patients with LVNC, this transition does not occur, leading to the development of a thickened, NC endomyocardial layer with prominent trabeculations that are continuous with the LV cavity and lack communication with the epicardial circulation.
The most widely used echocardiographic criteria, described by Jenni et al include (i) a thick, two-layered myocardium, with a thin, normally compacted layer and a markedly thickened non-compacted layer, with a ratio of NC/C >2, (ii) excessively prominent trabeculations, and (iii) deep inter-trabecular recesses filled by blood on color Doppler, measured at end-systole in the parasternal short-axis views.4 The major limitations associated with echocardiography in LVNC diagnosis include the identification of abnormal trabeculations and their accurate assessment. Abnormal trabeculations can be mistaken for normal myocardial trabeculations, false tendons, aberrant bands, cardiac tumors, and LV apical thrombi. Three-dimensional and contrast echocardiography are promising techniques in the morphological evaluation of LVNC. Novel echocardiographic techniques such as tissue Doppler, speckle tracking and strain rate imaging are also helpful in the evaluation of LVNC. Cardiac MRI can be used in patients in whom the apex is difficult to visualize with echocardiography or the echocardiographic diagnosis is doubtful. An NC/C ratio >2.3 measured at end-diastole is the most accepted criterion for cardiac MRI.5 In the absence of a gold standard, it may be difficult to differentiate LVNC from dilated cardiomyopathy and hypertrophic cardiomyopathy. In the latter conditions, the ratio between trabeculated and normal myocardium does not reach more than two. Moreover, the trabeculated regions associated with LVNC tend to be segmental rather than diffuse. Another differentiating feature is the trabeculations in LVNC are at the apex, lateral, or inferior walls of the LV, while the trabeculations of normal hearts course from the free wall to the ventricular septum.
Patients with a history of arrhythmias and repeat admissions for HF are more likely to have severe LV systolic dysfunction and poor prognosis. The anesthetic and perioperative considerations for patients with LVNC have not been studied in detail. The factors that increase sympathetic tone, afterload, or risk of arrhythmia, should be avoided.6 Myocardial oxygen balance should be maintained in patients with severe CAD. The treatment of HF in patients with LVNC follows the general guidelines for HF treatment. Meanwhile, the patients with NYHA class III or IV symptoms despite receiving optimal medical therapy, stage C or D HF, LVEF <35>120 ms, should receive an implantable cardioverter-defibrillator as a Class I recommendation.7 If HF is refractory to medical and device therapies, heart transplantation should be considered.8
In conclusion, LVNC is being increasingly diagnosed in clinical practice, although the current diagnostic criteria alone may be inadequate to confirm the diagnosis. A multimodality imaging approach and genetic testing may improve diagnostic accuracy. The clinical presentation of LVNC is highly variable, and there is no specific therapy for LVNC, and management depends on the clinical manifestations. A successful surgical outcome could be achieved in patients with severe CAD despite severe LV dysfunction.