AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/ 10.31579/2641-0427/010
1 Orthopaedics Department, Delhi Municipal Corporation Medical College & Hindu Rao Hospital, Delhi, India
*Corresponding Author: Suman Jain , Orthopaedics Department NDMC Medical College & Hindu Rao Hospital, Delhi, India.
Citation: Suman Jain and GBS Kiran. “The effects of different cell-based therapies at a critical time point during the soft-tissue healing process”, J. Orthopaedics and Surgical Sports Medicine. 1(2); Doi: 10.31579/2641-0427/010.
Copyright: © 2018 Suman Jain. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 08 October 2018 | Accepted: 27 October 2018 | Published: 30 October 2018
Keywords: Mesenchymal Stem Cells; Platelet Rich Plasma; Medial Collateral Ligament; Soft-tissue
Background: Cell-based therapy for soft tissue injuries remains controversial. Adult mesenchymal stem cells (MSCs) are therapeutic candidates given their capacity for self-renewal, immunoprivilege, and differentiation capacity for chondrocyte and tenocyte lineages. Platelet rich plasma (PRP) has been reported to promote collagen synthesis and cell proliferation, influencing the healing of ligaments and cartilage. We hypothesize that allogeneic MSCs and PRP have additive effects on promoting ligament healing in an in-vivo rat medial collateral ligament (MCL) injury model.
Methods: MCLs of 20 females Sprague rats were bilaterally transected and treated with either saline (controls) or 1 of 3 treatment groups; (1) allogeneic MSCs (105 cells), (2) PRP and (3) allogeneic MSCs & PRP. In addition, five rats were used for the Sham group (surgery + no ligament injury). Rats were sacrificed two weeks post-surgery and the MCLs harvested for histological analysis by hematoxylin and eosin and alcian blue staining. Statistical analysis was performed using Fischer’s exact test with pair-wise comparisons and Bonferroni multiple comparison correction.
Results: Histologically, differences across all injured groups (treatment groups and controls) were observed in cellularity (p < 0.0185), regeneration of collagen fibers (p < 0.0084), vascularity (p = 0.0129), inflammation (p = 0.0121) and glycosaminoglycan content (p = 0.0085). From pair-wise comparisons, only the combination allogeneic MSCs & PRP group differed significantly from controls in increased cellularity (p = 9.04 x 10-4) and regeneration of collagen fibers (p = 6.58x10-4). In addition, the PRP group showed significant increase in glycosaminoglycan (p = 0.006) content when compared to the allogeneic MSCs group.
Conclusion: The addition of allogeneic MSCs and PRP to an injured MCL show a significant histological increase in degree of cellularity, vascularity and the regeneration of collagen fibers when compared to controls. These data support a possible additive effect of combining allogeneic MSCs and PRP therapy to increase important repair factors during the proliferation/repair phase of post ligament injury. This preliminary study demonstrates that additional functional and biomechanical studies are warranted to determine the role that inflammatory responses versus tissue regeneration are contributing to this mechanism.
Abbreviations
MSCs: Mesenchymal Stem Cells; PRP: Platelet Rich Plasma; MCL: Medial Collateral Ligament; BM: Bone Marrow; SYN: Synovial Membrane; GFP: Green Fluorescent Protein; MEM: Minimum Essential Medium; PBS: Phosphate Buffered Saline; ACP: Autologous Conditioned Plasma system; H&E: Hematoxylin and Eosin; GAG: Glycosaminoglycan; ECM: Extracellular Matrix; SAS: Statistical Analysis Software; TGF-β: Transforming Growth Factor beta; VEGF: Vascular Endothelial Growth Factor.
In recent years, regenerative cell therapy for soft tissue injuries such as ligaments has generated wide-spread interest in the field of orthopedics. Soft tissue injuries are often problematic because of the limited ability of the tissue to self-repair. These injuries commonly result in the formation of inferior scar tissue and can cause a decrease in both function and performance of the affected area [1]. Regenerative cell based therapy aims to promote healthy tissue repair by providing the necessary elements, i.e. cells, growth factors and environment [1].
Adult mesenchymal stem cells (MSCs) have received considerable attention in soft tissue repair because of their high capacity for self-renewal and multipotency to differentiate into chondrocytes and tenocytes [2-8]. Also, MSCs migrate chemotactically to injured tissue and secrete cytokines with anti-inflammatory effects [6,7]. Adult MSCs can be isolated from several tissues including bone marrow (BM), synovial membrane (SYN), adipose and periosteum [2]. Of those BM and SYN MSCs have shown the greatest potential to repair soft tissue defects [5]. In a previous study investigating the tissue regenerative capabilities of MSCs, Wantanabe et al. injected MSCs into transected rat MCLs and detected donor cells with spindle shape nuclei comparable to native fibroblasts [8]. In a similar study, Nishimori et al. found increase regeneration of collagen fibers when compared to controls in injured rat MCLs treated with MSCs [9].
Platelet rich plasma (PRP) is platelet enriched blood plasma that contains several growth factors and cytokines with the potential to promote collagen synthesis and cell proliferation, thereby enhancing tissue repair [10-15]. Numerous studies have examined the potential healing effects of PRP therapy on soft tissue injury [16-18]. Although animal studies evaluating PRP therapy for cartilage and ligament injuries have shown promising effects, large scale controlled human clinical trials have yet to produce consistent results making the efficacy of PRP treatment for these injuries still up for debate [12,15].
The MCL is the most commonly injured knee ligament [19]. Its native healing ability often permits nonsurgical treatment, however, the repair process can take several years, and the healed ligament may never fully recover to its original mechanical function [20,21]. Despite this most patients achieve excellent results in terms of returning to play and normal ligament function with non-surgical management. The MCL’s healing properties suggest a potential role for cell-based/blood therapies to improve the ligament’s repair. Thus we are able to affectively investigate the MCL’s healing process following application of one of the above-mentioned regenerative therapies. Whether MSCs and PRP have an additive effect on soft-tissue repair when applied together is not known. The purpose of the present study is to investigate the histological effects of MSCs, PRP and their combined treatment application to ligament repair using a well-established rat MCL model [8,9,20,22,23]. We will examine whether there is histological evidence of an increased healing response when combining allogeneic MSCs and PRP therapy for soft tissue injuries. We hypothesize that combining MSCs and PRP treatment will have an additive effect towards ligament healing.
Isolations of MSCs
Bone marrow was obtained from the femurs and tibias of one adult green fluorescent protein (GFP) transgenic Sprague-Dawley male rat. Briefly, after flushing femur and tibia cells were plated in culture dishes containing Minimum Essential Medium (MEM) alpha supplemented with L-glutamine, ribonucleosides and deoxyribonucleosides (Life Technologies, Carlsbad, CA), 20
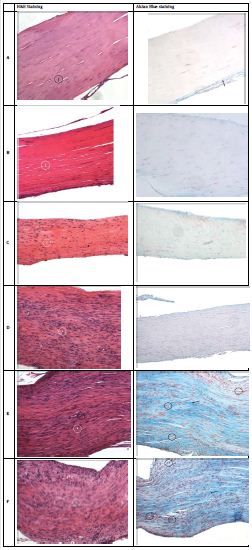
No complications were observed following surgery or throughout the observation period. Post-surgery, rats did not exhibit any signs of ligamentous injury such as a limp or altered gait. Data demographics are shown in table 4. H&E slides were analyzed for degree of cellularity, change in collagen, vascularity, and inflammation. Sham group received grades of 0 for all variables indicating normal ligament tissue.
Spearman correlation coefficients for the measured variables revealed that increase cellularity and regeneration of collagen fibers were highly correlated (r = 0.996). The remaining variables demonstrated either mild ) or moderate) correlation to each other (Table 5).
Treatment groups (MSCs, PRP, MSCs & PRP) and controls demonstrated histological differences in early ligament healing across all graded variables relative to a healthy medial collateral ligament. () Fisher’s exact test indicated significant histological differences during the proliferative/repair phase of ligament healing among injury groups (Controls, MSCs, MSCs & PRP, PRP) in degree of cellularity, regeneration of collagen fibers, degree of vascularity and inflammation (; across all groups). Pairwise comparisons revealed the allogeneic MSCs group differed nominally from controls in inflammation and degree of vascularity. For the PRP group, nominal significance when compared to controls was observed in degree of cellularity, regeneration of collagen fibers, inflammation, and vascularity. The combination allogeneic MSCs & PRP group demonstrated nominal histological significant differences in both inflammation and vascularity when compared to controls (See Table 2). Following correction for multiple comparisons, only the combined MSCs & PRP group reached significance histologically for degree of cellularity (p = 9.04 x10-4) and regeneration of collagen fibers (p = 6.58x10-4) when compared to controls during the proliferative/repair phase of ligament healing (Table 2). Treatment groups did not differ significantly from each other for any of the above variables (Table 3). The area of tissue involved in the healing process was significantly different among injured groups (p = 0.0021) with PRP and combination allogeneic MSCs & PRP having a significantly greater area of tissue involved then controls (p < 0>).
Alcian blue staining was used to determine histological changes in the glycosaminoglycan (GAG) content of the extracellular matrix (ECM) (Figure 1). All treatment groups and controls demonstrated on average an increase in GAG content within the extracellular matrix compared to a healthy MCL (Figure 1). Fischer’s exact test revealed significant differences in GAG content among injured groups (p = 0.0085). In addition the PRP group demonstrated nominal significance in increase glycosaminoglycan content when compared to controls (Table 2). Treatment groups did not differ significantly with respect to GAG content except the PRP group, which reached significance in increased GAG content when compared to MSCs alone (p = 0.006) (Table 3).
The purpose of this study was to to evaluate using a rat MCL model the histological effects of three different therapies intended to promote soft tissue healing during the early phases of ligament regeneration. Previous in-vivo studies investigating the healing properties of combining MSCs and PRP therapy have focused mainly on cartilage or bone defects. Our animal study looks at the potential additive effects at the histological level of combining allogeneic BM derived MSCs and PRP therapy for soft tissue healing. This current study examines early phases of ligament healing ≤ 14 ± 2 days), particularly the proliferative or regenerative/repair phase, enabling the examination of the basic histological changes that occur early on in the ligament tissue, thereby providing key insight and focus for future cell based therapy studies. Previous studies have used autogenic MSCs and employed a different type of injury [27]. Instead, in the current study we used an established rat MCL injury model to create a complete disruption of the MCL ligament. These characteristics make the results of this study distinct from previous studies [8,9,20,23].
Although a ruptured MCL has the ability to heal, prior studies have indicated inferior mechanical properties in the healed ligament one year after injury [22,29]. Previously, normal healing of the rat MCL was demonstrated to be an inflammatory driven process that resulted in inferior scar-like tissue [20,30]. The healing process is described to have three overlapping phases; inflammatory (day 0-day 5), proliferative or regenerative/repair phase (day 3-day 21) and remodeling (day 14-21-months) [20,31]. During the initial phases an influx of growth factors, cytokines and blood vessels are observed that help promote the healing process. Additionally, the proliferative phase or regenerative/repair phase demonstrates an influx in glycoproteins, proteoglycans and fibroblasts for collagen formation. These deposited collagen fibers mark the cornerstone of the regenerated tissue, maturing and organizing during the remodeling phase [32]. These attributes make the proliferative/repair phase a key time point to assess the early effects and regenerative properties of the various cell-based treatments. Additionally, the correlation of graded variables within the study emphasizes that although independent, these biological processes work together as part of the larger soft-tissue healing response.
Only the combination allogeneic MSCs & PRP group histologically displayed (p < 0>) an increased degree of cellularity and regeneration of collagen fibers when compared to controls (Cellularity p-value = 9.04x10-4, Collagen p-value = 6.58x10-4) (Table 2). Both MSCs and PRP therapy look to prevent the formation of scar tissue by promoting healthy tissue regeneration through various mechanisms. In addition to MSCs’ differentiation and direct engraftment ability, MSCs also have important paracrine effects. Implanted MSCs have been shown to increase the secretion of a variety of cytokines and growth factors and attract other local and distant cells from the host [33]. These signals stimulate various internal cells, playing important roles in promoting tissue recovery. Although in our study the MSCs were harvested from the bone marrow of an adult male GFP Sprague-Dawley rat we were not able to detect GFP 14 ± 2 days post-injury with immunofluorescent staining. While previous studies have indicated difficulty staining for GFP in transgenic mice [29,34], this suggests the possibility the tissue effects seen in the MSCs groups may be a result of paracrine effects instead of direct differentiation. Indeed, therapeutic benefits in organ damage have been seen with MSCs therapy without any evidence of sustained engraftment [33].
Previously, Martino et al found that injured sheep tendons treated with PRP, autogenic MSCs or both also demonstrated an increase in collagen when compared to controls [27]. These data support a possible additive effect of combining therapies (MSC & PRP) on the early stages of ligament healing.
There are several potential benefits for using allogeneic MSCs versus autogenic MSCs for cell-based therapies. The use of donor cells spares the patients from the risks and discomforts of cell-harvesting procedures from the iliac crest and also allows for collection of MSCs from optimal donors [35]. Recent studies have concluded that the allogeneic MSCs showed no significant alloimmune reactions and found the safety profile to be equivalent between the two groups [36]. Additionally using allogeneic MSCs/PRP has the potential to standardize the quantity and quality of these products, improving outcomes [37]. Furthermore producing these cells can be prepared ahead of time allowing immediate application when needed by patients making the clinical application of MSCs for tissue regeneration in orthopaedic medicine more readily achievable [38].
When treatment groups were compared to each other no individual treatment groups differed significantly (p > 0.008) from another with respect to cellularity, collagen fibers, vascularity or inflammation. Due to the similar processes induced by both therapies, it is possible that a larger sample size is needed to fully observe statically significant differences between treatment groups. One area that did display significant differences among treatment groups was the extent of glycosaminoglycan (GAG) content within the extracellular matrix (ECM). The PRP group displayed statistically significant increased GAG content when compared to the MSCs treatment group (p = 0.006). PRP was indicated previously to stimulate matrix biosynthesis in chondrocytes [36-39]. Following injury, GAGs help regulate inflammatory cell function and contribute to fibrogenesis [18,41-44]. Changes in GAG content have also been associated with scar formation and degenerative tissue [45-47]. These data suggest differences in the healing response stimulated by each therapy.
There are several limitations within this animal model. A surgically transected rat MCL is not equivalent to a torn human MCL; however, the biological processes are similar suggesting the results to be applicable for human MCL injury [20]. Although we found no changes in gait after transection of the MCL, the biological changes observed were compatible with significant injury. Previous studies have shown that the remodeling phase of the rat MCL healing process extends months past injury. Our results show potentially positive effects of combining MSCs and PRP during the early stages of ligament healing; however, a larger study with a longer observation period would be helpful to follow the healing process to its completion. This could help determine whether the regenerative tissue is of normal ligament tissue quality or of that of scar tissue. In addition, a study with multiple sacrificial time points would be helpful to determine at what point during the healing process does combining MSCs & PRP therapy demonstrates the most benefits toward soft-tissue healing. Although the focus of this study was to examine changes at the histological level, a study with biomechanical testing at the completion of the healing process would allow one to assess the overall functional behavior of the regenerative ligament tissue.
There are a variety of PRP formulations currently used in both clinical practice and research due in combination to individual variability of platelet concentration as well as different commercially available PRP centrifuge protocols. There is currently no consensus of what the optimal PRP concentration is for tissue regeneration. Both in vivo and in vitro studies have demonstrated healing benefits using PRP therapy with a variety of platelet concentrations (2x-6x baseline) [37,48]. The PRP protocol used for this study (Arthrex (ACP) Double Syringe centrifuge system™ (Arthex, Inc, Naples, Florida)) employs a plasma-base method to create Autologous Conditioned Plasma (ACP), a PRP formulation with generally 2x-3x baseline platelet concentration [49]. This specific protocol was applied because of the familiarity with it in our clinical practice.
In conclusion, this was a histological study that focused on the effects of different cell-based therapies at a critical time point during the soft-tissue healing process. While preliminary, these data suggest that at the peak of the proliferative/regenerative & repair phase of after ligament injury, combining MSCs and PRP therapy significantly increases cellularity and regeneration of collagen fibers when compared to controls and Sham ligaments. In addition, PRP influences GAG content within the ECM during the post-injury process. We have shown that combining allogeneic MSCs and PRP therapy leads to a significant histological response during the post-injury process. This preliminary study demonstrates that additional functional and biomechanical studies are warranted to determine the extent that inflammatory response versus tissue regeneration is contributing to the findings reported here. Collectively, these data support the potential benefit of cell-based therapies for soft tissue injuries and the translations of these therapies into clinical care.